DSE promotes aggressive glioma cell phenotypes by enhancing HB-EGF/ErbB signaling
- PMID: 29864158
- PMCID: PMC5986151
- DOI: 10.1371/journal.pone.0198364
DSE promotes aggressive glioma cell phenotypes by enhancing HB-EGF/ErbB signaling
Abstract
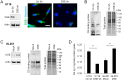
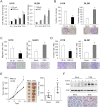
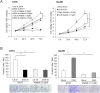
Remodeling of the extracellular matrix (ECM) in the tumor microenvironment promotes glioma progression. Chondroitin sulfate (CS) proteoglycans appear in the ECM and on the cell surface, and can be catalyzed by dermatan sulfate epimerase to form chondroitin sulfate/dermatan sulfate (CS/DS) hybrid chains. Dermatan sulfate epimerase 1 (DSE) is overexpressed in many types of cancer, and CS/DS chains mediate several growth factor signals. However, the role of DSE in gliomas has never been explored. In the present study, we determined the expression of DSE in gliomas by consulting a public database and conducting immunohistochemistry on a tissue array. Our investigation revealed that DSE was upregulated in gliomas compared with normal brain tissue. Furthermore, high DSE expression was associated with advanced tumor grade and poor survival. We found high DSE expression in several glioblastoma cell lines, and DSE expression directly mediated DS chain formation in glioblastoma cells. Knockdown of DSE suppressed the proliferation, migration, and invasion of glioblastoma cells. In contrast, overexpression of DSE in GL261 cells enhanced these malignant phenotypes and in vivo tumor growth. Interestingly, we found that DSE selectively regulated heparin-binding EGF-like growth factor (HB-EGF)-induced signaling in glioblastoma cells. Inhibiting epidermal growth factor receptor (EGFR) and ErbB2 with afatinib suppressed DSE-enhanced malignant phenotypes, establishing the critical role of the ErbB pathway in regulating the effects of DSE expression. This evidence indicates that upregulation of DSE in gliomas contributes to malignant behavior in cancer cells. We provide novel insight into the significance of DS chains in ErbB signaling and glioma pathogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
TRIM11 is overexpressed in high-grade gliomas and promotes proliferation, invasion, migration and glial tumor growth.Oncogene. 2013 Oct 17;32(42):5038-47. doi: 10.1038/onc.2012.531. Epub 2012 Nov 26. Oncogene. 2013. PMID: 23178488 Free PMC article.
-
Motility of glioblastoma cells is driven by netrin-1 induced gain of stemness.J Exp Clin Cancer Res. 2017 Jan 9;36(1):9. doi: 10.1186/s13046-016-0482-0. J Exp Clin Cancer Res. 2017. PMID: 28069038 Free PMC article.
-
Heparin-binding epidermal growth factor-like growth factor stimulates mitogenic signaling and is highly expressed in human malignant gliomas.Acta Neuropathol. 1998 Oct;96(4):322-8. doi: 10.1007/s004010050901. Acta Neuropathol. 1998. PMID: 9796995
-
Recent Advances in the Pathophysiology of Musculocontractural Ehlers-Danlos Syndrome.Genes (Basel). 2019 Dec 29;11(1):43. doi: 10.3390/genes11010043. Genes (Basel). 2019. PMID: 31905796 Free PMC article. Review.
-
Genetic heterogeneity and clinical variability in musculocontractural Ehlers-Danlos syndrome caused by impaired dermatan sulfate biosynthesis.Hum Mutat. 2015 May;36(5):535-47. doi: 10.1002/humu.22774. Epub 2015 Apr 6. Hum Mutat. 2015. PMID: 25703627 Review.
Cited by
-
Identifying common transcriptome signatures of cancer by interpreting deep learning models.Genome Biol. 2022 May 17;23(1):117. doi: 10.1186/s13059-022-02681-3. Genome Biol. 2022. PMID: 35581644 Free PMC article.
-
Comprehensive omics analyses profile genesets related with tumor heterogeneity of multifocal glioblastomas and reveal LIF/CCL2 as biomarkers for mesenchymal subtype.Theranostics. 2022 Jan 1;12(1):459-473. doi: 10.7150/thno.65739. eCollection 2022. Theranostics. 2022. PMID: 34987659 Free PMC article.
-
Dermatan Sulfate Affects Breast Cancer Cell Function via the Induction of Necroptosis.Cells. 2022 Jan 5;11(1):173. doi: 10.3390/cells11010173. Cells. 2022. PMID: 35011734 Free PMC article.
-
Upregulated proteoglycan-related signaling pathways in fluid flow shear stress-treated podocytes.Am J Physiol Renal Physiol. 2020 Aug 1;319(2):F312-F322. doi: 10.1152/ajprenal.00183.2020. Epub 2020 Jul 6. Am J Physiol Renal Physiol. 2020. PMID: 32628542 Free PMC article.
-
DSE inhibits melanoma progression by regulating tumor immune cell infiltration and VCAN.Cell Death Discov. 2023 Oct 13;9(1):373. doi: 10.1038/s41420-023-01676-8. Cell Death Discov. 2023. PMID: 37833287 Free PMC article.
References
-
- Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-oncology. 2017;19(suppl_5):v1–v88. doi: 10.1093/neuonc/nox158 . - DOI - PMC - PubMed
-
- Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. Jama. 2013;310(17):1842–50. doi: 10.1001/jama.2013.280319 . - DOI - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352(10):987–96. doi: 10.1056/NEJMoa043330 . - DOI - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013 . - DOI - PubMed
-
- Munkley J, Elliott DJ. Hallmarks of glycosylation in cancer. Onco_target. 2016;7(23):35478–89. doi: 10.18632/onco_target.8155 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

