Influence of Absorbable Calcium Sulfate-Based Bone Substitute Materials on Human Haemostasis-In Vitro Biological Behavior of Antibiotic Loaded Implants
- PMID: 29865173
- PMCID: PMC6025628
- DOI: 10.3390/ma11060935
Influence of Absorbable Calcium Sulfate-Based Bone Substitute Materials on Human Haemostasis-In Vitro Biological Behavior of Antibiotic Loaded Implants
Abstract
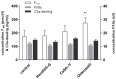
Calcium sulfate (CS) formulations are frequently implanted as antibiotically impregnated bone substitutes in orthopedic and trauma surgery to prevent or treat bone infections. Calcium ions have been discussed as candidates to accelerate blood coagulation. The goal of this study is to evaluate substance-specific influences of CS formulations on blood coagulation. Specific ELISAs were conducted to determine markers of activated blood coagulation after incubation of human blood with CS beads. Additionally, wettability with freshly drawn human blood was measured. Three different types of CS bone substitute beads were compared (CS dihydrate with tripalmitin, containing Gentamicin (Herafill®-G: Group A) or Vancomycin (CaSO₄-V: Group B); and a CS hemihydrate with Tobramycin (Osteoset®: Group C)). Examinations were performed by ELISA assays for F1+2, FXIIa and C3a. Our results prove that none of the CS preparations accelerated single specific assays for activated coagulation markers. This allows the conclusion that neither Herafill®-G (CaSO₄-G) nor CaSO₄-V alter haemostasis negatively. Blood samples incubated with Osteoset® display an elevated F1+2-activity. The addition of tripalmitin in Herafill®-G shifts the original into a significantly hydrophobic formulation. This was additionally proven by contact angle examination of the three substances with freshly drawn human blood, showing that acceleration of plasmatic coagulation is hindered by lipids and induced by surface effects caused by presence of rapidly soluble calcium ions in the Osteoset® preparation.
Keywords: C3a; F1+2; FXIIa; Herafill®-G; Osteoset®, gentamicin; calcium carbonate; calcium sulfate formulations; coagulation; in vitro; tobramycin; tripalmitin; vancomycin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Osteoinduction and -conduction through absorbable bone substitute materials based on calcium sulfate: in vivo biological behavior in a rabbit model.J Mater Sci Mater Med. 2018 Jan 9;29(2):17. doi: 10.1007/s10856-017-6017-1. J Mater Sci Mater Med. 2018. PMID: 29318379
-
Antimicrobial Formulations of Absorbable Bone Substitute Materials as Drug Carriers Based on Calcium Sulfate.Antimicrob Agents Chemother. 2016 Jun 20;60(7):3897-905. doi: 10.1128/AAC.00080-16. Print 2016 Jul. Antimicrob Agents Chemother. 2016. PMID: 27067337 Free PMC article.
-
Tobramycin exposure from active calcium sulfate bone graft substitute.BMC Pharmacol Toxicol. 2014 Mar 4;15:12. doi: 10.1186/2050-6511-15-12. BMC Pharmacol Toxicol. 2014. PMID: 24593819 Free PMC article. Clinical Trial.
-
Local antibiotic delivery with OsteoSet, DBX, and Collagraft.Clin Orthop Relat Res. 2006 Oct;451:29-33. doi: 10.1097/01.blo.0000229319.45416.81. Clin Orthop Relat Res. 2006. PMID: 16906070
-
Calcium carbonate powder containing gentamicin for mixing with bone grafts.Orthopedics. 2014 Aug;37(8):e669-72. doi: 10.3928/01477447-20140728-50. Orthopedics. 2014. PMID: 25102500
Cited by
-
Early Immune Response in Foreign Body Reaction Is Implant/Material Specific.Materials (Basel). 2022 Mar 16;15(6):2195. doi: 10.3390/ma15062195. Materials (Basel). 2022. PMID: 35329646 Free PMC article.
-
Limitations and modifications in the clinical application of calcium sulfate.Front Surg. 2024 Feb 29;11:1278421. doi: 10.3389/fsurg.2024.1278421. eCollection 2024. Front Surg. 2024. PMID: 38486794 Free PMC article. Review.
-
Aspect ratio-controlled preparation of α-CaSO4·0.5H2O from phosphogypsum in potassium tartrate aqueous solution.RSC Adv. 2019 Jul 11;9(38):21601-21607. doi: 10.1039/c9ra03569a. eCollection 2019 Jul 11. RSC Adv. 2019. PMID: 35518860 Free PMC article.
-
Impact of Gentamicin-Loaded Bone Graft on Defect Healing in a Sheep Model.Materials (Basel). 2019 Apr 4;12(7):1116. doi: 10.3390/ma12071116. Materials (Basel). 2019. PMID: 30987272 Free PMC article.
References
-
- Pforringer D., Obermeier A., Kiokekli M., Buchner H., Vogt S., Stemberger A., Burgkart R., Lucke M. Antimicrobial formulations of absorbable bone substitute materials as drug carriers based on calcium sulfate. Antimicrob. Agents Chemother. 2016;60:3897–3905. doi: 10.1128/AAC.00080-16. - DOI - PMC - PubMed
-
- Hashiguchi T. Basic concept for evaluating coagulation and fibrinolysis data. Rinsho Ketsueki. 2017;58:2096–2103. - PubMed
-
- Lalidou F., Kolios G., Drosos G.I. Bone infections and bone graft substitutes for local antibiotic therapy. Surg. Technol. Int. 2014;24:353–362. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

