Effectiveness and safety of long-term treatment with sulfonylureas in patients with neonatal diabetes due to KCNJ11 mutations: an international cohort study
- PMID: 29880308
- PMCID: PMC6058077
- DOI: 10.1016/S2213-8587(18)30106-2
Effectiveness and safety of long-term treatment with sulfonylureas in patients with neonatal diabetes due to KCNJ11 mutations: an international cohort study
Erratum in
-
Correction to Lancet Diabetes Endocrinol 2018; 6: 637-46.Lancet Diabetes Endocrinol. 2018 Sep;6(9):e17. doi: 10.1016/S2213-8587(18)30238-9. Lancet Diabetes Endocrinol. 2018. PMID: 30143191 Free PMC article. No abstract available.
Abstract
Background: KCNJ11 mutations cause permanent neonatal diabetes through pancreatic ATP-sensitive potassium channel activation. 90% of patients successfully transfer from insulin to oral sulfonylureas with excellent initial glycaemic control; however, whether this control is maintained in the long term is unclear. Sulfonylurea failure is seen in about 44% of people with type 2 diabetes after 5 years of treatment. Therefore, we did a 10-year multicentre follow-up study of a large international cohort of patients with KCNJ11 permanent neonatal diabetes to address the key questions relating to long-term efficacy and safety of sulfonylureas in these patients.
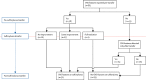
Methods: In this multicentre, international cohort study, all patients diagnosed with KCNJ11 permanent neonatal diabetes at five laboratories in Exeter (UK), Rome (Italy), Bergen (Norway), Paris (France), and Krakow (Poland), who transferred from insulin to oral sulfonylureas before Nov 30, 2006, were eligible for inclusion. Clinicians collected clinical characteristics and annual data relating to glycaemic control, sulfonylurea dose, severe hypoglycaemia, side-effects, diabetes complications, and growth. The main outcomes of interest were sulfonylurea failure, defined as permanent reintroduction of daily insulin, and metabolic control, specifically HbA1c and sulfonylurea dose. Neurological features associated with KCNJ11 permanent neonatal diabetes were also assessed. This study is registered with ClinicalTrials.gov, number NCT02624817.
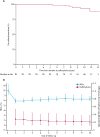
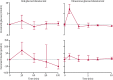
Findings: 90 patients were identified as being eligible for inclusion and 81 were enrolled in the study and provided long-term (>5·5 years cut-off) outcome data. Median follow-up duration for the whole cohort was 10·2 years (IQR 9·3-10·8). At most recent follow-up (between Dec 1, 2012, and Oct 4, 2016), 75 (93%) of 81 participants remained on sulfonylurea therapy alone. Excellent glycaemic control was maintained for patients for whom we had paired data on HbA1c and sulfonylurea at all time points (ie, pre-transfer [for HbA1c], year 1, and most recent follow-up; n=64)-median HbA1c was 8·1% (IQR 7·2-9·2; 65·0 mmol/mol [55·2-77·1]) before transfer to sulfonylureas, 5·9% (5·4-6·5; 41·0 mmol/mol [35·5-47·5]; p<0·0001 vs pre-transfer) at 1 year, and 6·4% (5·9-7·3; 46·4 mmol/mol [41·0-56·3]; p<0·0001 vs year 1) at most recent follow-up (median 10·3 years [IQR 9·2-10·9]). In the same patients, median sulfonylurea dose at 1 year was 0·30 mg/kg per day (0·14-0·53) and at most recent follow-up visit was 0·23 mg/kg per day (0·12-0·41; p=0·03). No reports of severe hypoglycaemia were recorded in 809 patient-years of follow-up for the whole cohort (n=81). 11 (14%) patients reported mild, transient side-effects, but did not need to stop sulfonylurea therapy. Seven (9%) patients had microvascular complications; these patients had been taking insulin longer than those without complications (median age at transfer to sulfonylureas 20·5 years [IQR 10·5-24·0] vs 4·1 years [1·3-10·2]; p=0·0005). Initial improvement was noted following transfer to sulfonylureas in 18 (47%) of 38 patients with CNS features. After long-term therapy with sulfonylureas, CNS features were seen in 52 (64%) of 81 patients.
Interpretation: High-dose sulfonylurea therapy is an appropriate treatment for patients with KCNJ11 permanent neonatal diabetes from diagnosis. This therapy is safe and highly effective, maintaining excellent glycaemic control for at least 10 years.
Funding: Wellcome Trust, Diabetes UK, Royal Society, European Research Council, Norwegian Research Council, Kristian Gerhard Jebsen Foundation, Western Norway Regional Health Authority, Southern and Eastern Norway Regional Health Authority, Italian Ministry of Health, Aide aux Jeunes Diabetiques, Societe Francophone du Diabete, Ipsen, Slovak Research and Development Agency, and Research and Development Operational Programme funded by the European Regional Development Fund.
Copyright © 2018 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Precision medicine in KCNJ11 permanent neonatal diabetes.Lancet Diabetes Endocrinol. 2018 Aug;6(8):594-595. doi: 10.1016/S2213-8587(18)30138-4. Epub 2018 Jun 4. Lancet Diabetes Endocrinol. 2018. PMID: 29880307 Free PMC article. No abstract available.
Similar articles
-
Molecular Genetics, Clinical Characteristics, and Treatment Outcomes of KATP-Channel Neonatal Diabetes Mellitus in Vietnam National Children's Hospital.Front Endocrinol (Lausanne). 2021 Sep 9;12:727083. doi: 10.3389/fendo.2021.727083. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34566892 Free PMC article.
-
Successful transfer to sulfonylureas in KCNJ11 neonatal diabetes is determined by the mutation and duration of diabetes.Diabetologia. 2016 Jun;59(6):1162-6. doi: 10.1007/s00125-016-3921-8. Epub 2016 Mar 31. Diabetologia. 2016. PMID: 27033559 Free PMC article.
-
Case report: Better late than never, but sooner is better: switch from CSII to sulfonylureas in two patients with neonatal diabetes due to KCNJ11 variants.Front Endocrinol (Lausanne). 2023 May 11;14:1143736. doi: 10.3389/fendo.2023.1143736. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37251668 Free PMC article.
-
Precision Medicine: Long-Term Treatment with Sulfonylureas in Patients with Neonatal Diabetes Due to KCNJ11 Mutations.Curr Diab Rep. 2019 Jun 27;19(8):52. doi: 10.1007/s11892-019-1175-9. Curr Diab Rep. 2019. PMID: 31250216 Free PMC article. Review.
-
Diabetes and hypoglycaemia in young children and mutations in the Kir6.2 subunit of the potassium channel: therapeutic consequences.Diabetes Metab. 2006 Dec;32(6):569-80. doi: 10.1016/S1262-3636(07)70311-7. Diabetes Metab. 2006. PMID: 17296510 Review.
Cited by
-
Safety and Efficacy of Using Advanced Hybrid Closed Loop Off-Label in an Infant Diagnosed with Permanent Neonatal Diabetes Mellitus: A Case Report and a Look to the Future.Children (Basel). 2024 Oct 9;11(10):1225. doi: 10.3390/children11101225. Children (Basel). 2024. PMID: 39457190 Free PMC article.
-
Long-Term Effects of Incretin-Based Drugs on Glycemic Control in Permanent Neonatal Diabetes.JCEM Case Rep. 2024 Oct 18;2(11):luae188. doi: 10.1210/jcemcr/luae188. eCollection 2024 Nov. JCEM Case Rep. 2024. PMID: 39430732 Free PMC article.
-
Successful Transition to Sulfonylurea for Relapsed Monogenic Diabetes Due to Rare 6q23.3 Duplication.JCEM Case Rep. 2024 Oct 16;2(10):luae180. doi: 10.1210/jcemcr/luae180. eCollection 2024 Oct. JCEM Case Rep. 2024. PMID: 39416272 Free PMC article.
-
Editorial: Personalized therapies for monogenic diabetes.Front Genet. 2024 Sep 27;15:1496367. doi: 10.3389/fgene.2024.1496367. eCollection 2024. Front Genet. 2024. PMID: 39399218 Free PMC article. No abstract available.
-
Insulin Delivery Technology for Treatment of Infants with Neonatal Diabetes Mellitus: A Systematic Review.Diabetes Ther. 2024 Nov;15(11):2293-2308. doi: 10.1007/s13300-024-01653-z. Epub 2024 Sep 18. Diabetes Ther. 2024. PMID: 39292435 Free PMC article. Review.
References
-
- Gloyn AL, Pearson ER, Antcliff JF. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–1849. - PubMed
-
- Sagen JV, Raeder H, Hathout E. Permanent neonatal diabetes due to mutations in KCNJ11 encoding Kir6.2: patient characteristics and initial response to sulfonylurea therapy. Diabetes. 2004;53:2713–2718. - PubMed
-
- Zung A, Glaser B, Nimri R, Zadik Z. Glibenclamide treatment in permanent neonatal diabetes mellitus due to an activating mutation in Kir6.2. J Clin Endocrinol Metab. 2004;89:5504–5507. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

