Nicotinic Restoration of Excitatory Neuroplasticity Is Linked to Improved Implicit Motor Learning Skills in Deprived Smokers
- PMID: 29892258
- PMCID: PMC5985290
- DOI: 10.3389/fneur.2018.00367
Nicotinic Restoration of Excitatory Neuroplasticity Is Linked to Improved Implicit Motor Learning Skills in Deprived Smokers
Abstract
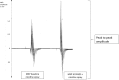
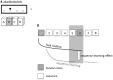
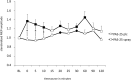
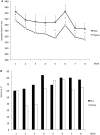
Nicotine has been shown to modulate neuroplasticity, cognition, and learning processes in smokers and non-smokers. A possible mechanism for its effect on learning and memory formation is its impact on long-term depression and long-term potentiation (LTP). Nicotine abstinence in smokers is often correlated with impaired cognitive performance. As neuroplasticity is closely connected to learning and memory formation, we aimed to explore the effect of nicotine spray administration in deprived smokers on paired-associative stimulation (PAS25)-induced neuroplasticity and on performance of the serial reaction time task (SRTT), a sequential motor learning paradigm. Deprived smokers (n = 12) under placebo medication displayed reduced excitatory neuroplasticity induced by PAS25. Plasticity was restored by nicotine spray administration. Likewise, SRTT-performance improved after nicotine spray administration compared to placebo administration (n = 19). The results indicate a restitutional effect of nicotine spray in deprived smokers on both: LTP-like neuroplasticity and motor learning. These results present a possible explanation for persistence of nicotine addiction and probability of relapse.
Keywords: cognition; neuroplasticity; nicotine; non-invasive brain stimulation; smokers.
Figures

Similar articles
-
Diverging effects of nicotine on motor learning performance: Improvement in deprived smokers and attenuation in non-smokers.Addict Behav. 2017 Nov;74:90-97. doi: 10.1016/j.addbeh.2017.05.017. Epub 2017 May 24. Addict Behav. 2017. PMID: 28600927
-
Nicotine modulates human brain plasticity via calcium-dependent mechanisms.J Physiol. 2018 Nov;596(22):5429-5441. doi: 10.1113/JP276502. Epub 2018 Oct 17. J Physiol. 2018. PMID: 30218585 Free PMC article.
-
Compromised neuroplasticity in cigarette smokers under nicotine withdrawal is restituted by the nicotinic α4β2-receptor partial agonist varenicline.Sci Rep. 2017 May 3;7(1):1387. doi: 10.1038/s41598-017-01428-6. Sci Rep. 2017. PMID: 28469204 Free PMC article.
-
Do smokers self-administer pure nicotine? A review of the evidence.Psychopharmacology (Berl). 2004 Apr;173(1-2):18-26. doi: 10.1007/s00213-004-1781-2. Epub 2004 Mar 5. Psychopharmacology (Berl). 2004. PMID: 15004737 Review.
-
Transcranial cerebellar direct current stimulation (tcDCS): motor control, cognition, learning and emotions.Neuroimage. 2014 Jan 15;85 Pt 3:918-23. doi: 10.1016/j.neuroimage.2013.04.122. Epub 2013 May 9. Neuroimage. 2014. PMID: 23664951 Review.
Cited by
-
The two-way relationship between nicotine and cortical activity: a systematic review of neurobiological and treatment aspects.Eur Arch Psychiatry Clin Neurosci. 2021 Feb;271(1):157-180. doi: 10.1007/s00406-020-01155-6. Epub 2020 Jun 27. Eur Arch Psychiatry Clin Neurosci. 2021. PMID: 32594235
-
The Influence of Recreational Substance Use in TMS Research.Brain Sci. 2020 Oct 18;10(10):751. doi: 10.3390/brainsci10100751. Brain Sci. 2020. PMID: 33080965 Free PMC article. Review.
-
Effect of Paired Associative Stimulation on Corticomotor Excitability in Chronic Smokers.Brain Sci. 2019 Mar 15;9(3):62. doi: 10.3390/brainsci9030062. Brain Sci. 2019. PMID: 30875969 Free PMC article.
-
Nicotine Facilitates Facial Stimulation-Evoked Mossy Fiber-Granule Cell Long-Term Potentiation in vivo in Mice.Front Cell Neurosci. 2022 Jul 4;16:905724. doi: 10.3389/fncel.2022.905724. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35860314 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

