Battle of the sexes: contrasting roles of testis-specific protein Y-encoded (TSPY) and TSPX in human oncogenesis
- PMID: 29974883
- PMCID: PMC6498724
- DOI: 10.4103/aja.aja_43_18
Battle of the sexes: contrasting roles of testis-specific protein Y-encoded (TSPY) and TSPX in human oncogenesis
Abstract
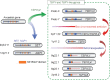
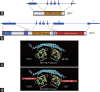
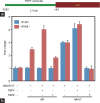
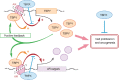
The Y-located testis-specific protein Y-encoded (TSPY) and its X-homologue TSPX originated from the same ancestral gene, but act as a proto-oncogene and a tumor suppressor gene, respectively. TSPY has specialized in male-specific functions, while TSPX has assumed the functions of the ancestral gene. Both TSPY and TSPX harbor a conserved SET/NAP domain, but are divergent at flanking structures. Specifically, TSPX contains a C-terminal acidic domain, absent in TSPY. They possess contrasting properties, in which TSPY and TSPX, respectively, accelerate and arrest cell proliferation, stimulate and inhibit cyclin B-CDK1 phosphorylation activities, have no effect and promote proteosomal degradation of the viral HBx oncoprotein, and exacerbate and repress androgen receptor (AR) and constitutively active AR variant, such as AR-V7, gene transactivation. The inhibitory domain has been mapped to the carboxyl acidic domain in TSPX, truncation of which results in an abbreviated TSPX exerting positive actions as TSPY. Transposition of the acidic domain to the C-terminus of TSPY results in an inhibitory protein as intact TSPX. Hence, genomic mutations/aberrant splicing events could generate TSPX proteins with truncated acidic domain and oncogenic properties as those for TSPY. Further, TSPY is upregulated by AR and AR-V7 in ligand-dependent and ligand-independent manners, respectively, suggesting the existence of a positive feedback loop between a Y-located proto-oncogene and male sex hormone/receptors, thereby amplifying the respective male oncogenic actions in human cancers and diseases. TSPX counteracts such positive feedback loop. Hence, TSPY and TSPX are homologues on the sex chromosomes that function at the two extremes of the human oncogenic spectrum.
Keywords: AR-V7; TSPX; TSPY; androgen receptor; cell cycle regulation; cyclin B-CDK1; oncogene; sex chromosomes; sex differences; tumor suppressor.
Figures

Similar articles
-
The Y-located proto-oncogene TSPY exacerbates and its X-homologue TSPX inhibits transactivation functions of androgen receptor and its constitutively active variants.Hum Mol Genet. 2017 Mar 1;26(5):901-912. doi: 10.1093/hmg/ddx005. Hum Mol Genet. 2017. PMID: 28169398 Free PMC article.
-
TSPY and its X-encoded homologue interact with cyclin B but exert contrasting functions on cyclin-dependent kinase 1 activities.Oncogene. 2008 Oct 16;27(47):6141-50. doi: 10.1038/onc.2008.206. Epub 2008 Jun 30. Oncogene. 2008. PMID: 18591933
-
The potential contributions of a Y-located protooncogene and its X homologue in sexual dimorphisms in hepatocellular carcinoma.Hum Pathol. 2014 Sep;45(9):1847-58. doi: 10.1016/j.humpath.2014.05.002. Epub 2014 May 23. Hum Pathol. 2014. PMID: 25017435
-
Gonadoblastoma locus and the TSPY gene on the human Y chromosome.Birth Defects Res C Embryo Today. 2009 Mar;87(1):114-22. doi: 10.1002/bdrc.20144. Birth Defects Res C Embryo Today. 2009. PMID: 19306348 Review.
-
Structure and function of TSPY, the Y-chromosome gene coding for the "testis-specific protein".Cytogenet Cell Genet. 1998;80(1-4):209-13. doi: 10.1159/000014982. Cytogenet Cell Genet. 1998. PMID: 9678360 Review.
Cited by
-
Analysis of novel enzalutamide-resistant cells: upregulation of testis-specific Y-encoded protein gene promotes the expression of androgen receptor splicing variant 7.Transl Cancer Res. 2020 Oct;9(10):6232-6245. doi: 10.21037/tcr-20-1463. Transl Cancer Res. 2020. PMID: 35117234 Free PMC article.
-
A Multi-Omics Study of Human Testis and Epididymis.Molecules. 2021 Jun 2;26(11):3345. doi: 10.3390/molecules26113345. Molecules. 2021. PMID: 34199411 Free PMC article.
-
The Role of Cell Division Autoantigen 1 (CDA1) in Renal Fibrosis of Diabetic Nephropathy.Biomed Res Int. 2021 Apr 28;2021:6651075. doi: 10.1155/2021/6651075. eCollection 2021. Biomed Res Int. 2021. PMID: 33997036 Free PMC article. Review.
-
Y chromosome in health and diseases.Cell Biosci. 2020 Aug 13;10:97. doi: 10.1186/s13578-020-00452-w. eCollection 2020. Cell Biosci. 2020. PMID: 32817785 Free PMC article. Review.
-
Y chromosome is moving out of sex determination shadow.Cell Biosci. 2022 Jan 4;12(1):4. doi: 10.1186/s13578-021-00741-y. Cell Biosci. 2022. PMID: 34983649 Free PMC article. Review.
References
-
- Hughes JF, Rozen S. Genomics and genetics of human and primate Y chromosomes. Annu Rev Genomics Hum Genet. 2012;13:83–108. - PubMed
-
- Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423:825–37. - PubMed
-
- Hughes JF, Page DC. The history of the Y chromosome in man. Nat Genet. 2016;48:588–9. - PubMed
-
- Lyon MF. X-chromosome inactivation. Curr Biol. 1999;9:R235–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

