The roles of neutrophil serine proteinases in idiopathic inflammatory myopathies
- PMID: 29976235
- PMCID: PMC6034343
- DOI: 10.1186/s13075-018-1632-x
The roles of neutrophil serine proteinases in idiopathic inflammatory myopathies
Abstract
Background: Dermatomyositis and polymyositis are the best known idiopathic inflammatory myopathies (IIMs). Classic histopathologic findings include the infiltration of inflammatory cells into muscle tissues. Neutrophil serine proteinases (NSPs) are granule-associated enzymes and play roles in inflammatory cell migration by increasing the permeability of vascular endothelial cells. In this study, we aimed to find the roles of NSPs in pathogenesis of IIMs.
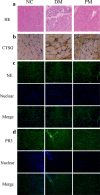
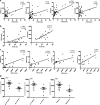
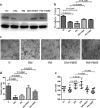
Methods: RNA and DNA were isolated to measure the relative expression of NSPs and their methylation levels. The expression of NSPs in serum and muscle tissues was tested by enzyme-linked immunosorbent assay, immunohistochemistry, and immunofluorescence, respectively. Serum from patients was used to culture the human dermal microvascular endothelial cells (HDMECs), and then we observed the influence of serum on expression of VE-cadherin, endothelial cell tube formation, and transendothelial migration of peripheral blood mononuclear cells (PBMCs).
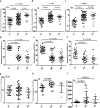
Results: We found that the expression of NSPs was increased in PBMCs, serum, and muscle tissues of IIM patients; these NSPs were hypomethylated in the PBMCs of patients. Serum NSPs were positively correlated with clinical indicators of IIM patients, including lactic dehydrogenase, erythrocyte sedimentation rate, C-reactive protein, immunoglobulin G, immunoglobulin M, and immunoglobulin A. Patients with anti-Jo-1, with anti-Ro-52, or without interstitial lung disease had lower levels of proteinase 3. Serum NSPs degraded the VE-cadherin of HDMECs, and serum NSP application increased the permeability of HDMECs.
Conclusions: Our studies indicate, for the first time, that NSPs play an important role in muscle inflammatory cell infiltration by increasing the permeability of vascular endothelial cells in IIM patients.
Keywords: Dermatomyositis; Inflammatory cell migration; Neutrophil serine proteinases; Polymyositis; Vascular permeability.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the institutional review board at Xiangya Hospital, Central South University of Changsha (Changsha, Hunan, China). All of the participants in the study signed a written informed consent form prior to participation.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Neutrophil serine proteases exert proteolytic activity on endothelial cells.Kidney Int. 2015 Oct;88(4):764-75. doi: 10.1038/ki.2015.159. Epub 2015 Jun 10. Kidney Int. 2015. PMID: 26061547
-
Correlation of PMN elastase and PMN elastase-to-neutrophil ratio with disease activity in patients with myositis.J Transl Med. 2019 Dec 16;17(1):420. doi: 10.1186/s12967-019-02176-z. J Transl Med. 2019. PMID: 31842908 Free PMC article.
-
Endothelial cell SHP-2 negatively regulates neutrophil adhesion and promotes transmigration by enhancing ICAM-1-VE-cadherin interaction.FASEB J. 2017 Nov;31(11):4759-4769. doi: 10.1096/fj.201700280R. Epub 2017 Jul 12. FASEB J. 2017. PMID: 28701303 Free PMC article.
-
Dendritic cells and the immunopathogenesis of idiopathic inflammatory myopathies.Curr Opin Rheumatol. 2008 Nov;20(6):669-74. doi: 10.1097/BOR.0b013e3283157538. Curr Opin Rheumatol. 2008. PMID: 18946326 Review.
-
Idiopathic Inflammatory Myopathies: A Review of the Classification and Impact of Pathogenesis.Int J Mol Sci. 2017 May 18;18(5):1084. doi: 10.3390/ijms18051084. Int J Mol Sci. 2017. PMID: 28524083 Free PMC article. Review.
Cited by
-
Transcriptome Sequencing Identifies PLAUR as an Important Player in Patients With Dermatomyositis-Associated Interstitial Lung Disease.Front Genet. 2021 Dec 6;12:784215. doi: 10.3389/fgene.2021.784215. eCollection 2021. Front Genet. 2021. PMID: 34938325 Free PMC article.
-
Pathogenic role and clinical significance of neutrophils and neutrophil extracellular traps in idiopathic inflammatory myopathies.Clin Exp Med. 2024 May 30;24(1):115. doi: 10.1007/s10238-024-01384-2. Clin Exp Med. 2024. PMID: 38814339 Free PMC article. Review.
-
Stromal vascular fraction in the treatment of myositis.Cell Death Discov. 2023 Sep 19;9(1):346. doi: 10.1038/s41420-023-01605-9. Cell Death Discov. 2023. PMID: 37726262 Free PMC article. Review.
-
Exosomal miRNAs in autoimmune skin diseases.Front Immunol. 2023 Dec 1;14:1307455. doi: 10.3389/fimmu.2023.1307455. eCollection 2023. Front Immunol. 2023. PMID: 38106405 Free PMC article. Review.
-
The Role of Immune Cells in the Pathogenesis of Idiopathic Inflammatory Myopathies.Aging Dis. 2021 Feb 1;12(1):247-260. doi: 10.14336/AD.2020.0410. eCollection 2021 Feb. Aging Dis. 2021. PMID: 33532139 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

