Physiological signature of a novel potentiator of AMPA receptor signalling
- PMID: 30044951
- PMCID: PMC6525152
- DOI: 10.1016/j.mcn.2018.07.003
Physiological signature of a novel potentiator of AMPA receptor signalling
Abstract
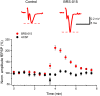
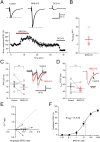
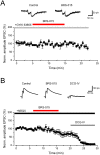
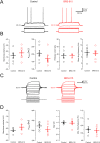
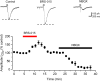
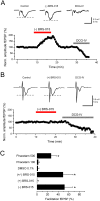
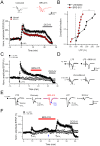
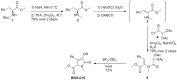
We have synthesized a novel small molecule based on the pyrrolidinone-containing core structure of clausenamide, which is a candidate anti-dementia drug. The synthetic route yielded multi-gram quantities of an isomeric racemate mixture in a short number of steps. When tested in hippocampal slices from young adult rats the compound enhanced AMPA receptor-mediated signalling at mossy fibre synapses, and potentiated inward currents evoked by local application of l-glutamate onto CA3 pyramidal neurons. It facilitated the induction of mossy fibre LTP, but the magnitude of potentiation was smaller than that observed in untreated slices. The racemic mixture was separated and it was shown that only the (-) enantiomer was active. Toxicity analysis indicated that cell lines tolerated the compound at concentrations well above those enhancing synaptic transmission. Our results unveil a small molecule whose physiological signature resembles that of a potent nootropic drug.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Induction of mossy fiber --> Ca3 long-term potentiation requires translocation of synaptically released Zn2+.J Neurosci. 2001 Oct 15;21(20):8015-25. doi: 10.1523/JNEUROSCI.21-20-08015.2001. J Neurosci. 2001. PMID: 11588174 Free PMC article.
-
Synapse-specific compartmentalization of signaling cascades for LTP induction in CA3 interneurons.Neuroscience. 2015 Apr 2;290:332-45. doi: 10.1016/j.neuroscience.2015.01.024. Epub 2015 Jan 28. Neuroscience. 2015. PMID: 25637803 Free PMC article.
-
Nobiletin, a citrus flavonoid with neurotrophic action, augments protein kinase A-mediated phosphorylation of the AMPA receptor subunit, GluR1, and the postsynaptic receptor response to glutamate in murine hippocampus.Eur J Pharmacol. 2008 Jan 14;578(2-3):194-200. doi: 10.1016/j.ejphar.2007.09.028. Epub 2007 Oct 2. Eur J Pharmacol. 2008. PMID: 17976577
-
Kainate receptors and the induction of mossy fibre long-term potentiation.Philos Trans R Soc Lond B Biol Sci. 2003 Apr 29;358(1432):657-66. doi: 10.1098/rstb.2002.1216. Philos Trans R Soc Lond B Biol Sci. 2003. PMID: 12740111 Free PMC article. Review.
-
Role of giant depolarizing potentials in shaping synaptic currents in the developing hippocampus.Crit Rev Neurobiol. 2006;18(1-2):13-23. doi: 10.1615/critrevneurobiol.v18.i1-2.30. Crit Rev Neurobiol. 2006. PMID: 17725505 Review.
References
-
- Arai A., Lynch G. AMPA receptor desensitization modulates synaptic responses induced by repetitive afferent stimulation in hippocampal slices. Brain Res. 1998;799:235–242. - PubMed
-
- Arai A.C., Kessler M., Rogers G., Lynch G. Effects of the potent ampakine CX614 on hippocampal and recombinant AMPA receptors: interactions with cyclothiazide and GYKI 52466. Mol. Pharmacol. 2000;58:802–813. - PubMed
-
- Baude A., Nusser Z., Molnar E., McIlhinney R.A., Somogyi P. High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience. 1995;69:1031–1055. - PubMed
-
- Castillo P.E., Malenka R.C., Nicoll R.A. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

