JNK regulates muscle remodeling via myostatin/SMAD inhibition
- PMID: 30072727
- PMCID: PMC6072737
- DOI: 10.1038/s41467-018-05439-3
JNK regulates muscle remodeling via myostatin/SMAD inhibition
Abstract
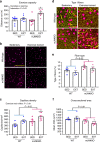
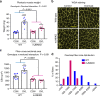
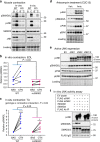
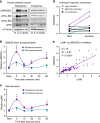
Skeletal muscle has a remarkable plasticity to adapt and remodel in response to environmental cues, such as physical exercise. Endurance exercise stimulates improvements in muscle oxidative capacity, while resistance exercise induces muscle growth. Here we show that the c-Jun N-terminal kinase (JNK) is a molecular switch that when active, stimulates muscle fibers to grow, resulting in increased muscle mass. Conversely, when muscle JNK activation is suppressed, an alternative remodeling program is initiated, resulting in smaller, more oxidative muscle fibers, and enhanced aerobic fitness. When muscle is exposed to mechanical stress, JNK initiates muscle growth via phosphorylation of the transcription factor, SMAD2, at specific linker region residues leading to inhibition of the growth suppressor, myostatin. In human skeletal muscle, this JNK/SMAD signaling axis is activated by resistance exercise, but not endurance exercise. We conclude that JNK acts as a key mediator of muscle remodeling during exercise via regulation of myostatin/SMAD signaling.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Myostatin is a key mediator between energy metabolism and endurance capacity of skeletal muscle.Am J Physiol Regul Integr Comp Physiol. 2014 Aug 15;307(4):R444-54. doi: 10.1152/ajpregu.00377.2013. Epub 2014 Jun 25. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24965795
-
Inhibition of myostatin signaling through Notch activation following acute resistance exercise.PLoS One. 2013 Jul 2;8(7):e68743. doi: 10.1371/journal.pone.0068743. Print 2013. PLoS One. 2013. PMID: 23844238 Free PMC article.
-
Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways.Endocrinology. 2010 Feb;151(2):628-38. doi: 10.1210/en.2009-1177. Epub 2009 Dec 18. Endocrinology. 2010. PMID: 20022929 Free PMC article.
-
PI3 kinase regulation of skeletal muscle hypertrophy and atrophy.Curr Top Microbiol Immunol. 2010;346:267-78. doi: 10.1007/82_2010_78. Curr Top Microbiol Immunol. 2010. PMID: 20593312 Review.
-
Caveolin-3 regulates myostatin signaling. Mini-review.Acta Myol. 2008 Jul;27(1):19-24. Acta Myol. 2008. PMID: 19108573 Free PMC article. Review.
Cited by
-
The ties that bind: functional clusters in limb-girdle muscular dystrophy.Skelet Muscle. 2020 Jul 29;10(1):22. doi: 10.1186/s13395-020-00240-7. Skelet Muscle. 2020. PMID: 32727611 Free PMC article. Review.
-
Exercise Therapy for People With Sarcopenic Obesity: Myokines and Adipokines as Effective Actors.Front Endocrinol (Lausanne). 2022 Feb 17;13:811751. doi: 10.3389/fendo.2022.811751. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35250869 Free PMC article. Review.
-
HER2-driven breast cancer suppression by the JNK signaling pathway.Proc Natl Acad Sci U S A. 2023 Jan 24;120(4):e2218373120. doi: 10.1073/pnas.2218373120. Epub 2023 Jan 19. Proc Natl Acad Sci U S A. 2023. PMID: 36656864 Free PMC article.
-
ZAKβ is activated by cellular compression and mediates contraction-induced MAP kinase signaling in skeletal muscle.EMBO J. 2022 Sep 1;41(17):e111650. doi: 10.15252/embj.2022111650. Epub 2022 Jul 28. EMBO J. 2022. PMID: 35899396 Free PMC article.
-
JNK signaling contributes to skeletal muscle wasting and protein turnover in pancreatic cancer cachexia.Cancer Lett. 2020 Oct 28;491:70-77. doi: 10.1016/j.canlet.2020.07.025. Epub 2020 Jul 28. Cancer Lett. 2020. PMID: 32735910 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

