The Spectrum of Fundamental Basic Science Discoveries Contributing to Organismal Aging
- PMID: 30075061
- PMCID: PMC6327947
- DOI: 10.1002/jbmr.3564
The Spectrum of Fundamental Basic Science Discoveries Contributing to Organismal Aging
Abstract
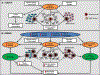
Aging research has undergone unprecedented advances at an accelerating rate in recent years, leading to excitement in the field as well as opportunities for imagination and innovation. Novel insights indicate that, rather than resulting from a preprogrammed series of events, the aging process is predominantly driven by fundamental non-adaptive mechanisms that are interconnected, linked, and overlap. To varying degrees, these mechanisms also manifest with aging in bone where they cause skeletal fragility. Because these mechanisms of aging can be manipulated, it might be possible to slow, delay, or alleviate multiple age-related diseases and their complications by _targeting conserved genetic signaling pathways, controlled functional networks, and basic biochemical processes. Indeed, findings in various mammalian species suggest that _targeting fundamental aging mechanisms (eg, via either loss-of-function or gain-of-function mutations or administration of pharmacological therapies) can extend healthspan; ie, the healthy period of life free of chronic diseases. In this review, we summarize the evidence supporting the role of the spectrum of fundamental basic science discoveries contributing to organismal aging, with emphasis on mammalian studies and in particular aging mechanisms in bone that drive skeletal fragility. These mechanisms or aging hallmarks include: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. Because these mechanisms are linked, interventions that ameliorate one hallmark can in theory ameliorate others. In the field of bone and mineral research, current challenges include defining the relative contributions of each aging hallmark to the natural skeletal aging process, better understanding the complex interconnections among the hallmarks, and identifying the most effective therapeutic strategies to safely _target multiple hallmarks. Based on their interconnections, it may be feasible to simultaneously interfere with several fundamental aging mechanisms to alleviate a wide spectrum of age-related chronic diseases, including osteoporosis. © 2018 American Society for Bone and Mineral Research.
Keywords: AGING; BONE; DISEASE PREVENTION; OSTEOPOROSIS.
© 2018 American Society for Bone and Mineral Research.
Figures
Similar articles
-
The hallmarks of aging.Cell. 2013 Jun 6;153(6):1194-217. doi: 10.1016/j.cell.2013.05.039. Cell. 2013. PMID: 23746838 Free PMC article. Review.
-
Hallmarks of aging: An expanding universe.Cell. 2023 Jan 19;186(2):243-278. doi: 10.1016/j.cell.2022.11.001. Epub 2023 Jan 3. Cell. 2023. PMID: 36599349 Review.
-
Magnesium and the Hallmarks of Aging.Nutrients. 2024 Feb 9;16(4):496. doi: 10.3390/nu16040496. Nutrients. 2024. PMID: 38398820 Free PMC article. Review.
-
Sedentary behavior and the biological hallmarks of aging.Ageing Res Rev. 2023 Jan;83:101807. doi: 10.1016/j.arr.2022.101807. Epub 2022 Nov 22. Ageing Res Rev. 2023. PMID: 36423885 Review.
-
New hallmarks of ageing: a 2022 Copenhagen ageing meeting summary.Aging (Albany NY). 2022 Aug 29;14(16):6829-6839. doi: 10.18632/aging.204248. Epub 2022 Aug 29. Aging (Albany NY). 2022. PMID: 36040386 Free PMC article.
Cited by
-
A proteomics approach to study mouse long bones: examining baseline differences and mechanical loading-induced bone formation in young-adult and old mice.Aging (Albany NY). 2024 Oct 12;16(19):12726-12768. doi: 10.18632/aging.206131. Epub 2024 Oct 12. Aging (Albany NY). 2024. PMID: 39400554 Free PMC article.
-
Cellular senescence and the skeleton: pathophysiology and therapeutic implications.J Clin Invest. 2022 Feb 1;132(3):e154888. doi: 10.1172/JCI154888. J Clin Invest. 2022. PMID: 35104801 Free PMC article. Review.
-
Cellular senescence in age-related disorders.Transl Res. 2020 Dec;226:96-104. doi: 10.1016/j.trsl.2020.06.007. Epub 2020 Jun 20. Transl Res. 2020. PMID: 32569840 Free PMC article. Review.
-
Transcriptional regulation of cyclophilin D by BMP/Smad signaling and its role in osteogenic differentiation.Elife. 2022 May 30;11:e75023. doi: 10.7554/eLife.75023. Elife. 2022. PMID: 35635445 Free PMC article.
-
Age Differences in Motor Recruitment Patterns of the Shoulder in Dynamic and Isometric Contractions. A Cross-Sectional Study.J Clin Med. 2021 Feb 2;10(3):525. doi: 10.3390/jcm10030525. J Clin Med. 2021. PMID: 33540507 Free PMC article.
References
-
- Williams GC. Pleitropy, natural selection, and the evolution of senescence. Evolution. 1957;11(4):398–411.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

