Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial
- PMID: 30098152
- PMCID: PMC6248083
- DOI: 10.1001/jamaoncol.2018.3039
Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial
Erratum in
-
Errors in Number at Risk and Author Email Address.JAMA Oncol. 2018 Nov 1;4(11):1625. doi: 10.1001/jamaoncol.2018.5345. JAMA Oncol. 2018. PMID: 30419091 Free PMC article. No abstract available.
Abstract
Importance: Anlotinib is a novel multi_target tyrosine kinase inhibitor for tumor angiogenesis and proliferative signaling. A phase 2 trial showed anlotinib to improve progression-free survival with a potential benefit of overall survival, leading to the phase 3 trial to confirm the drug's efficacy in advanced non-small cell lung cancer (NSCLC).
Objective: To investigate the efficacy of anlotinib on overall survival of patients with advanced NSCLC progressing after second-line or further treatment.
Design, setting, and participants: The ALTER 0303 trial was a multicenter, double-blind, phase 3 randomized clinical trial designed to evaluate the efficacy and safety of anlotinib in patients with advanced NSCLC. Patients from 31 grade-A tertiary hospitals in China were enrolled between March 1, 2015, and August 31, 2016. Those aged 18 to 75 years who had histologically or cytologically confirmed NSCLC were eligible (n = 606), and those who had centrally located squamous cell carcinoma with cavitary features or brain metastases that were uncontrolled or controlled for less than 2 months were excluded. Patients (n = 440) were randomly assigned in a 2-to-1 ratio to receive either 12 mg/d of anlotinib or a matched placebo. All cases were treated with study drugs at least once in accordance with the intention-to-treat principle.
Main outcomes and measures: The primary end point was overall survival. The secondary end points were progression-free survival, objective response rate, disease control rate, quality of life, and safety.
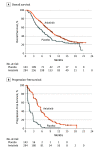
Results: In total, 439 patients were randomized, 296 to the anlotinib group (106 [36.1%] were female and 188 [64.0%] were male, with a mean [SD] age of 57.9 [9.1] years) and 143 to the placebo group (46 [32.2%] were female and 97 [67.8%] were male, with a mean [SD] age of 56.8 [9.1] years). Overall survival was significantly longer in the anlotinib group (median, 9.6 months; 95% CI, 8.2-10.6) than the placebo group (median, 6.3 months; 95% CI, 5.0-8.1), with a hazard ratio (HR) of 0.68 (95% CI, 0.54-0.87; P = .002). A substantial increase in progression-free survival was noted in the anlotinib group compared with the placebo group (median, 5.4 months [95% CI, 4.4-5.6] vs 1.4 months [95% CI, 1.1-1.5]; HR, 0.25 [95% CI, 0.19-0.31]; P < .001). Considerable improvement in objective response rate and disease control rate was observed in the anlotinib group over the placebo group. The most common grade 3 or higher adverse events in the anlotinib arm were hypertension and hyponatremia.
Conclusions and relevance: Among the Chinese patients in this trial, anlotinib appears to lead to prolonged overall survival and progression-free survival. This finding suggests that anlotinib is well tolerated and is a potential third-line or further therapy for patients with advanced NSCLC.
Trial registration: ClinicalTrials.gov identifier: NCT02388919.
Conflict of interest statement
Figures

Similar articles
-
Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302).Br J Cancer. 2018 Mar 6;118(5):654-661. doi: 10.1038/bjc.2017.478. Epub 2018 Feb 13. Br J Cancer. 2018. PMID: 29438373 Free PMC article. Clinical Trial.
-
Quality of life results from a randomized, double-blinded, placebo-controlled, multi-center phase III trial of anlotinib in patients with advanced non-small cell lung cancer.Lung Cancer. 2018 Aug;122:32-37. doi: 10.1016/j.lungcan.2018.05.013. Epub 2018 May 18. Lung Cancer. 2018. PMID: 30032842 Clinical Trial.
-
China National Medical Products Administration approval summary: anlotinib for the treatment of advanced non-small cell lung cancer after two lines of chemotherapy.Cancer Commun (Lond). 2019 Jun 20;39(1):36. doi: 10.1186/s40880-019-0383-7. Cancer Commun (Lond). 2019. PMID: 31221221 Free PMC article. Review.
-
Effect of anlotinib as a third- or further-line therapy in advanced non-small cell lung cancer patients with different histologic types: Subgroup analysis in the ALTER0303 trial.Cancer Med. 2020 Apr;9(8):2621-2630. doi: 10.1002/cam4.2913. Epub 2020 Feb 16. Cancer Med. 2020. PMID: 32064794 Free PMC article. Clinical Trial.
-
Anlotinib: First Global Approval.Drugs. 2018 Jul;78(10):1057-1062. doi: 10.1007/s40265-018-0939-x. Drugs. 2018. PMID: 29943374 Review.
Cited by
-
Equivalent efficacy study of QL1101 and bevacizumab on untreated advanced non-squamous non-small cell lung cancer patients: a phase 3 randomized, double-blind clinical trial.Cancer Biol Med. 2021 Mar 12;18(3):816-24. doi: 10.20892/j.issn.2095-3941.2020.0212. Online ahead of print. Cancer Biol Med. 2021. PMID: 33710815 Free PMC article.
-
Triple therapy boosts survival in NSCLC patients with brain metastases: a retrospective cohort study of chemotherapy, ICIs, and antiangiogenic agents.Cancer Immunol Immunother. 2024 Sep 6;73(11):226. doi: 10.1007/s00262-024-03797-0. Cancer Immunol Immunother. 2024. PMID: 39237636 Free PMC article.
-
NEAT 1 knockdown enhances the sensitivity of human non-small-cell lung cancer cells to anlotinib.Aging (Albany NY). 2021 May 12;13(10):13941-13953. doi: 10.18632/aging.203004. Epub 2021 May 12. Aging (Albany NY). 2021. PMID: 33982669 Free PMC article.
-
Trial Watch: experimental TLR7/TLR8 agonists for oncological indications.Oncoimmunology. 2020 Jul 21;9(1):1796002. doi: 10.1080/2162402X.2020.1796002. Oncoimmunology. 2020. PMID: 32934889 Free PMC article. Review.
-
Stevens-Johnson syndrome induced by toripalimab in a previously EGFR-TKI-treated advanced lung adenocarcinoma patient harboring EGFR mutations 19 del/T790M/C797S in trans and cis: a case report.Front Pharmacol. 2023 Nov 14;14:1131703. doi: 10.3389/fphar.2023.1131703. eCollection 2023. Front Pharmacol. 2023. PMID: 38035001 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

