The polyphenol resveratrol promotes skeletal growth in mice through a sirtuin 1-bone morphogenic protein 2 longevity axis
- PMID: 30125963
- PMCID: PMC6177622
- DOI: 10.1111/bph.14477
The polyphenol resveratrol promotes skeletal growth in mice through a sirtuin 1-bone morphogenic protein 2 longevity axis
Abstract
Background and purpose: The polyphenol resveratrol (RSV) exists in high quantities in certain foods (e.g. grapes and nuts). However, the capacity of RSV to confer physiological health benefits and a biological mechanism through which this might occur remains unclear.
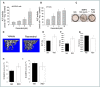
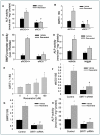
Experimental approach: Aged, RSV-treated (300 mg·kg-1 ·day-1 ) and genetically modified [endothelial NOS (eNOS-/- )] female mice were assessed using histomorphometric and μCT analysis. Alongside in vivo analysis, molecular siRNA knockdown and pharmacological manipulation of eNOS, BMP2 and sirtuin 1 (SIRT1) and functional cellular assays in an osteoblast cell line panel, explored the mechanism through which RSV might impact overall bone volume.
Key results: RSV promoted osteoblast activity and bone growth in vivo. RSV dose-dependently and simultaneously increased alkaline phosphatase (ALP) and eNOS levels. Similarly, NO-donor treatment increased ALP, runt homology transcription factor 2, BMP2 and stimulated bone formation, whilst eNOS-deficient mice displayed a bone loss phenotype. Moreover, RSV-induced increase in ALP and BMP2 expression was blocked in eNOS-/- osteoblasts and by BMP-inhibitor noggin. The longevity-linked SIRT1 enzyme was positively regulated by RSV and SIRT1 deletion reduced eNOS, BMP2 and ALP. Like eNOS deletion, loss of SIRT1 blocked RSV-induced osteoblast activity; however, SIRT1 levels remained unchanged in eNOS-/- mice, indicating RSV activation of SIRT1 stimulates BMP2 release via eNOS. This signalling axis is supported by decreased SIRT1, eNOS and BMP2 confirmed in old versus young bone.
Conclusions and implications: These findings suggest a new mechanism of action in bone remodelling and the ageing skeleton, where RSV positively impacts bone homeostasis via SIRT1 activation of BMP2.
© 2018 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures


Similar articles
-
Beneficial effect of resveratrol on phenotypic features and activity of osteoarthritic osteoblasts.Arthritis Res Ther. 2017 Jun 30;19(1):151. doi: 10.1186/s13075-017-1365-2. Arthritis Res Ther. 2017. PMID: 28666466 Free PMC article.
-
Resveratrol Mitigates High-Fat Diet-Induced Vascular Dysfunction by Activating the Akt/eNOS/NO and Sirt1/ER Pathway.J Cardiovasc Pharmacol. 2018 Nov;72(5):231-241. doi: 10.1097/FJC.0000000000000621. J Cardiovasc Pharmacol. 2018. PMID: 30399060
-
Effect of a Resveratrol/Quercetin Mixture on the Reversion of Hypertension Induced by a Short-Term Exposure to High Sucrose Levels Near Weaning and a Long-Term Exposure That Leads to Metabolic Syndrome in Rats.Int J Mol Sci. 2020 Mar 23;21(6):2231. doi: 10.3390/ijms21062231. Int J Mol Sci. 2020. PMID: 32210194 Free PMC article.
-
The Potential of Resveratrol to Act as a Caloric Restriction Mimetic Appears to Be Limited: Insights from Studies in Mice.Adv Nutr. 2021 Jun 1;12(3):995-1005. doi: 10.1093/advances/nmaa148. Adv Nutr. 2021. PMID: 33271594 Free PMC article. Review.
-
Resveratrol and endothelial function: A literature review.Pharmacol Res. 2021 Aug;170:105725. doi: 10.1016/j.phrs.2021.105725. Epub 2021 Jun 10. Pharmacol Res. 2021. PMID: 34119624 Review.
Cited by
-
The Impact of Hempseed Consumption on Bone Parameters and Body Composition in Growing Female C57BL/6 Mice.Int J Environ Res Public Health. 2022 May 11;19(10):5839. doi: 10.3390/ijerph19105839. Int J Environ Res Public Health. 2022. PMID: 35627377 Free PMC article.
-
A neuronal action of sirtuin 1 suppresses bone mass in young and aging mice.J Clin Invest. 2022 Dec 1;132(23):e152868. doi: 10.1172/JCI152868. J Clin Invest. 2022. PMID: 36194488 Free PMC article.
-
Epigenetic regulation of bone remodeling by natural compounds.Pharmacol Res. 2019 Sep;147:104350. doi: 10.1016/j.phrs.2019.104350. Epub 2019 Jul 14. Pharmacol Res. 2019. PMID: 31315065 Free PMC article. Review.
-
Key Signaling Pathways in Aging and Potential Interventions for Healthy Aging.Cells. 2021 Mar 16;10(3):660. doi: 10.3390/cells10030660. Cells. 2021. PMID: 33809718 Free PMC article. Review.
-
Role of Dietary Polyphenols in the Activity and Expression of Nitric Oxide Synthases: A Review.Antioxidants (Basel). 2023 Jan 7;12(1):147. doi: 10.3390/antiox12010147. Antioxidants (Basel). 2023. PMID: 36671009 Free PMC article. Review.
References
-
- Aguirre J, Buttery L, O'shaughnessy M, Afzal F, Fernandez De Marticorena I et al (2001). Endothelial nitric oxide synthase gene‐deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am J Pathol 158: 247–257. - PMC - PubMed
-
- Bellaver B, Souza DG, Bobermin LD, Souza DO, Goncalves CA, Quincozes‐Santos A (2015). Resveratrol protects hippocampal astrocytes against LPS‐induced neurotoxicity through HO‐1, p38 and ERK pathways. Neurochem Res 40: 1600–1608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

