Prenatal Steroids and Metabolic Dysfunction: Lessons from Sheep
- PMID: 30192640
- PMCID: PMC7451114
- DOI: 10.1146/annurev-animal-020518-115154
Prenatal Steroids and Metabolic Dysfunction: Lessons from Sheep
Abstract
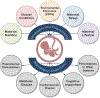
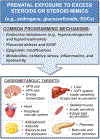
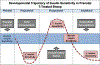
Prenatal exposure to excess steroids or steroid mimics can disrupt the normal developmental trajectory of organ systems, culminating in adult disease. The metabolic system is particularly susceptible to the deleterious effects of prenatal steroid excess. Studies in sheep demonstrate that prenatal exposure to excess native steroids or endocrine-disrupting chemicals with steroidogenic activity, such as bisphenol A, results in postnatal development of numerous cardiometabolic perturbations, including insulin resistance, increased adiposity, altered adipocyte size and distribution, and hypertension. The similarities in the phenotypic outcomes programmed by these different prenatal insults suggest that common mechanisms may be involved, and these may include hormonal imbalances (e.g., hyperandrogenism and hyperinsulinemia), oxidative stress, inflammation, lipotoxicity, and epigenetic alterations. Animal models, including the sheep, provide mechanistic insight into the metabolic repercussions associated with prenatal steroid exposure and represent valuable research tools in understanding human health and disease. Focusing on the sheep model, this review summarizes the cardiometabolic perturbations programmed by prenatal exposure to different native steroids and steroid mimics and discusses the potential mechanisms underlying the development of adverse outcomes.
Keywords: cardiometabolic disease; developmental programming; sheep; steroid hormones.
Figures
Similar articles
-
Developmental reprogramming of reproductive and metabolic dysfunction in sheep: native steroids vs. environmental steroid receptor modulators.Int J Androl. 2010 Apr;33(2):394-404. doi: 10.1111/j.1365-2605.2009.01024.x. Epub 2010 Jan 12. Int J Androl. 2010. PMID: 20070410 Free PMC article. Review.
-
Steroidogenic versus Metabolic Programming of Reproductive Neuroendocrine, Ovarian and Metabolic Dysfunctions.Neuroendocrinology. 2015;102(3):226-37. doi: 10.1159/000381830. Epub 2015 Apr 1. Neuroendocrinology. 2015. PMID: 25832114 Free PMC article. Review.
-
Reproduction Symposium: developmental programming of reproductive and metabolic health.J Anim Sci. 2014 Aug;92(8):3199-210. doi: 10.2527/jas.2014-7637. J Anim Sci. 2014. PMID: 25074449 Free PMC article. Review.
-
Developmental Programming: Impact of Gestational Steroid and Metabolic Milieus on Mediators of Insulin Sensitivity in Prenatal Testosterone-Treated Female Sheep.Endocrinology. 2017 Sep 1;158(9):2783-2798. doi: 10.1210/en.2017-00460. Endocrinology. 2017. PMID: 28911168 Free PMC article.
-
Developmental Programming: Impact of Gestational Steroid and Metabolic Milieus on Adiposity and Insulin Sensitivity in Prenatal Testosterone-Treated Female Sheep.Endocrinology. 2016 Feb;157(2):522-35. doi: 10.1210/en.2015-1565. Epub 2015 Dec 9. Endocrinology. 2016. PMID: 26650569 Free PMC article.
Cited by
-
Developmental programming: Prenatal testosterone excess disrupts pancreatic islet developmental trajectory in female sheep.Mol Cell Endocrinol. 2020 Dec 1;518:110950. doi: 10.1016/j.mce.2020.110950. Epub 2020 Jul 26. Mol Cell Endocrinol. 2020. PMID: 32726642 Free PMC article.
-
Prenatal cortisol exposure impairs adrenal function but not glucose metabolism in adult sheep.J Endocrinol. 2024 Jan 29;260(3):e230326. doi: 10.1530/JOE-23-0326. Print 2024 Mar 1. J Endocrinol. 2024. PMID: 38109257 Free PMC article.
-
Sex-Specific Perturbation of Systemic Lipidomic Profile in Newborn Lambs Impacted by Prenatal Testosterone Excess.Endocrinology. 2023 Dec 23;165(2):bqad187. doi: 10.1210/endocr/bqad187. Endocrinology. 2023. PMID: 38060679 Free PMC article.
-
Stress, Sex, and Sugar: Glucocorticoids and Sex-Steroid Crosstalk in the Sex-Specific Misprogramming of Metabolism.J Endocr Soc. 2020 Jul 3;4(8):bvaa087. doi: 10.1210/jendso/bvaa087. eCollection 2020 Aug 1. J Endocr Soc. 2020. PMID: 32734132 Free PMC article. Review.
-
Gestational testosterone excess early to mid-pregnancy disrupts maternal lipid homeostasis and activates biosynthesis of phosphoinositides and phosphatidylethanolamines in sheep.Sci Rep. 2024 Mar 14;14(1):6230. doi: 10.1038/s41598-024-56886-6. Sci Rep. 2024. PMID: 38486090 Free PMC article.
References
-
- Barker D 2004. The developmental origins of adult disease. J. Am. Coll. Nutr. 23:588S–95S - PubMed
-
- Fowden AL, Forhead AJ. 2004. Endocrine mechanisms of intrauterine programming. Reproduction 127:515–26 - PubMed
-
- Barker DJ. 2007. The origins of the developmental origins theory. J. Intern. Med. 261:412–17 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
