Transcriptome analysis and physiological responses of the potato plantlets in vitro under red, blue, and white light conditions
- PMID: 30221107
- PMCID: PMC6119552
- DOI: 10.1007/s13205-018-1410-0
Transcriptome analysis and physiological responses of the potato plantlets in vitro under red, blue, and white light conditions
Abstract
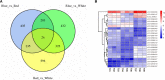
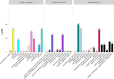
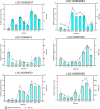
Light is an important factor for plant development and has serious effects on the growth, production and quality of potatoes. However, the physical and molecular mechanisms by which potato plantlets cope with different light qualities are not understood. In this study, the potato "Zhuanxinwu", which is a germplasm potato resource with a high anthocyanin content, was used for physiological and transcriptome profiling analyses to uncover the different mechanisms that occur in response to blue, red and white light conditions, with the white light condition serving as the control. Multiple growth indexes, protective enzyme activity and metabolite accumulation were measured. The results indicated that white light promoted a shift in biomass allocation away from tubers to leaves to enhance dry leaf matter and reduce tuber fresh/dry weight relative to the effects of blue or red light. The leaf area and anthocyanin content values were greater for plants grown in blue light than those grown in white or red light, suggesting that combinations of different spectra were more conducive to regulating potato growth. A total of 2220 differentially expressed genes (DEGs) were found among the three samples, and the DEGs in the three comparison sets were analyzed. A total of 1180 and 984 DEGs were identified in the red light (Red) and blue light (Blue) conditions compared to the control condition, respectively, and 359 DEGs overlapped between the two comparison sets (Blue_vs_White and Red_vs_White). Interestingly, the 24 most common overlapped DEGs were involved in photosynthesis, respiration, and reactive oxygen species (ROS) scavenging. Of these DEGs, four genes involved in photosynthesis and two genes involved in pigment synthesis were highly expressed, implying that some genes could be implemented to cope with different light spectra by regulating the expression of DEGs involved in the corresponding metabolic pathways. In conclusion, our study characterizes physiological responses of potato to different light qualities and identifies potential pathways and candidate genes involved in these responses, thus providing a basis for further research on artificial light regulation of potato plant growth.
Keywords: Different light spectra; Differentially expressed genes; Physiological response; Potato; RNA-seq.
Conflict of interest statement
Compliance with ethical standardsThe authors declare there is no conflict of interest.
Figures


Similar articles
-
Physiological responses and transcriptome analysis of Spirodela polyrhiza under red, blue, and white light.Planta. 2021 Dec 2;255(1):11. doi: 10.1007/s00425-021-03764-4. Planta. 2021. PMID: 34855030
-
An RNA-Seq Analysis of Grape Plantlets Grown in vitro Reveals Different Responses to Blue, Green, Red LED Light, and White Fluorescent Light.Front Plant Sci. 2017 Jan 31;8:78. doi: 10.3389/fpls.2017.00078. eCollection 2017. Front Plant Sci. 2017. PMID: 28197159 Free PMC article.
-
Production of Potato (Solanum tuberosum L.) Seed Tuber under Artificial LED Light Irradiation in Plant Factory.Plants (Basel). 2021 Feb 4;10(2):297. doi: 10.3390/plants10020297. Plants (Basel). 2021. PMID: 33557310 Free PMC article.
-
Integrated transcriptome and metabolome analysis reveals the anthocyanin biosynthesis mechanisms in blueberry (Vaccinium corymbosum L.) leaves under different light qualities.Front Plant Sci. 2022 Dec 8;13:1073332. doi: 10.3389/fpls.2022.1073332. eCollection 2022. Front Plant Sci. 2022. PMID: 36570935 Free PMC article.
-
Morpho-physio-biochemical, molecular, and phytoremedial responses of plants to red, blue, and green light: a review.Environ Sci Pollut Res Int. 2024 Mar;31(14):20772-20791. doi: 10.1007/s11356-024-32532-6. Epub 2024 Feb 23. Environ Sci Pollut Res Int. 2024. PMID: 38393568 Review.
Cited by
-
Transcriptomic Insights into Molecular Response of Butter Lettuce to Different Light Wavelengths.Plants (Basel). 2024 Jun 7;13(12):1582. doi: 10.3390/plants13121582. Plants (Basel). 2024. PMID: 38931014 Free PMC article.
-
RNA Sequencing Reveals That Both Abiotic and Biotic Stress-Responsive Genes are Induced during Expression of Steroidal Glycoalkaloid in Potato Tuber Subjected to Light Exposure.Genes (Basel). 2019 Nov 11;10(11):920. doi: 10.3390/genes10110920. Genes (Basel). 2019. PMID: 31718041 Free PMC article.
-
Transcriptome-based analysis of carotenoid accumulation-related gene expression in petals of Chinese cabbage (Brassica rapa L.).3 Biotech. 2019 Jul;9(7):274. doi: 10.1007/s13205-019-1813-6. Epub 2019 Jun 19. 3 Biotech. 2019. PMID: 31245238 Free PMC article.
References
-
- Basu S, Giri RK, Benazir I, Kumar S, Rajwanshi R, Dwivedi SK, Kumar G. Comprehensive physiological analyses and reactive oxygen species profiling in drought tolerant rice genotypes under salinity stress. Physiol Mol Biol Plants. 2017;23(4):837–850. doi: 10.1007/s12298-017-0477-0. - DOI - PMC - PubMed
-
- Birch PRJ, Bryan G, Fenton B, Gilroy EM, Hein I, Jones JT, Prashar A, Taylor MA, Torrance L, Toth IK. Crops that feed the world 8: potato: are the trends of increased global production sustainable? Food Secur. 2012;4(4):477–508. doi: 10.1007/s12571-012-0220-1. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

