CypA Regulates AIP4-Mediated M1 Ubiquitination of Influenza A Virus
- PMID: 30328013
- PMCID: PMC6235765
- DOI: 10.1007/s12250-018-0058-6
CypA Regulates AIP4-Mediated M1 Ubiquitination of Influenza A Virus
Abstract
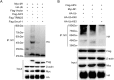
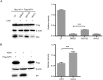
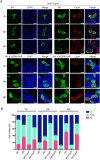
Cyclophilin A (CypA) is a peptidyl-prolyl cis/trans isomerase that interacts with the matrix protein (M1) of influenza A virus (IAV) and restricts virus replication by regulating the ubiquitin-proteasome-mediated degradation of M1. However, the mechanism by which CypA regulates M1 ubiquitination remains unknown. In this study, we reported that E3 ubiquitin ligase AIP4 promoted K48-linked ubiquitination of M1 at K102 and K104, and accelerated ubiquitin-proteasome-mediated degradation of M1. The recombinant IAV with mutant M1 (K102R/K104R) could not be rescued, suggesting that the ubiquitination of M1 at K102/K104 was essential for IAV replication. Furthermore, CypA inhibited AIP4-mediated M1 ubiquitination by impairing the interaction between AIP4 and M1. More importantly, both the mutations of M1 (K102R/K104R) and CypA inhibited the nuclear export of M1, indicating that CypA regulates the cellular localization of M1 via inhibition of AIP4-mediated M1 ubiquitination at K102 and K104, which results in the reduced replication of IAV. Collectively, our findings reveal a novel ubiquitination-based mechanism by which CypA regulates the replication of IAV.
Keywords: AIP4; Cyclophilin A (CypA); Influenza A virus (IAV); M1; Ubiquitination.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
Figures


Similar articles
-
PSMD12-Mediated M1 Ubiquitination of Influenza A Virus at K102 Regulates Viral Replication.J Virol. 2022 Aug 10;96(15):e0078622. doi: 10.1128/jvi.00786-22. Epub 2022 Jul 21. J Virol. 2022. PMID: 35861516 Free PMC article.
-
Cyclophilin A restricts influenza A virus replication through degradation of the M1 protein.PLoS One. 2012;7(2):e31063. doi: 10.1371/journal.pone.0031063. Epub 2012 Feb 8. PLoS One. 2012. PMID: 22347431 Free PMC article.
-
Insights into the roles of cyclophilin A during influenza virus infection.Viruses. 2013 Jan 15;5(1):182-91. doi: 10.3390/v5010182. Viruses. 2013. PMID: 23322171 Free PMC article. Review.
-
Nonproteolytic K29-Linked Ubiquitination of the PB2 Replication Protein of Influenza A Viruses by Proviral Cullin 4-Based E3 Ligases.mBio. 2020 Apr 7;11(2):e00305-20. doi: 10.1128/mBio.00305-20. mBio. 2020. PMID: 32265326 Free PMC article.
-
Cyclophilin A and viral infections.Biochem Biophys Res Commun. 2012 Aug 10;424(4):647-50. doi: 10.1016/j.bbrc.2012.07.024. Epub 2012 Jul 16. Biochem Biophys Res Commun. 2012. PMID: 22814107 Free PMC article. Review.
Cited by
-
Acetylation, Methylation and Allysine Modification Profile of Viral and Host Proteins during Influenza A Virus Infection.Viruses. 2021 Jul 20;13(7):1415. doi: 10.3390/v13071415. Viruses. 2021. PMID: 34372620 Free PMC article.
-
PSMD12-Mediated M1 Ubiquitination of Influenza A Virus at K102 Regulates Viral Replication.J Virol. 2022 Aug 10;96(15):e0078622. doi: 10.1128/jvi.00786-22. Epub 2022 Jul 21. J Virol. 2022. PMID: 35861516 Free PMC article.
-
PROTAC _targeting cyclophilin A controls virus-induced cytokine storm.iScience. 2023 Aug 3;26(9):107535. doi: 10.1016/j.isci.2023.107535. eCollection 2023 Sep 15. iScience. 2023. PMID: 37636080 Free PMC article.
-
Repurposing of cyclophilin A inhibitors as broad-spectrum antiviral agents.Drug Discov Today. 2022 Jul;27(7):1895-1912. doi: 10.1016/j.drudis.2022.05.016. Epub 2022 May 21. Drug Discov Today. 2022. PMID: 35609743 Free PMC article. Review.
-
Phosphorylation of Influenza A Virus Matrix Protein 1 at Threonine 108 Controls Its Multimerization State and Functional Association with the STRIPAK Complex.mBio. 2023 Feb 28;14(1):e0323122. doi: 10.1128/mbio.03231-22. Epub 2023 Jan 5. mBio. 2023. PMID: 36602306 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

