Long-term safety of pirfenidone: results of the prospective, observational PASSPORT study
- PMID: 30364407
- PMCID: PMC6194203
- DOI: 10.1183/23120541.00084-2018
Long-term safety of pirfenidone: results of the prospective, observational PASSPORT study
Abstract
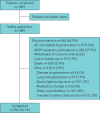
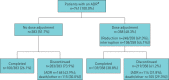
Real-world studies include a broader patient population for a longer duration than randomised controlled trials (RCTs) and can provide relevant insights for clinical practice. PASSPORT was a multicentre, prospective, post-authorisation study of patients who were newly prescribed pirfenidone and followed for 2 years after initiating treatment. Physicians collected data on adverse drug reactions (ADRs), serious ADRs (SADRs) and ADRs of special interest (ADRSI) at baseline and then every 3 months. Post hoc stepwise logistic regression models were used to identify baseline characteristics associated with discontinuing treatment due to an ADR. Patients (n=1009, 99.7% with idiopathic pulmonary fibrosis) had a median pirfenidone exposure of 442.0 days. Overall, 741 (73.4%) patients experienced ADRs, most commonly nausea (20.6%) and fatigue (18.5%). ADRs led to treatment discontinuation in 290 (28.7%) patients after a median of 99.5 days. Overall, 55 (5.5%) patients experienced SADRs, with a fatal outcome in six patients. ADRSI were reported in 693 patients, most commonly gastrointestinal symptoms (38.3%) and photosensitivity reactions/skin rashes (29.0%). Older age and female sex were associated with early treatment discontinuation due to an ADR. Findings were consistent with the known safety profile of pirfenidone, based on RCT data and other post-marketing experience, with no new safety signals observed.
Conflict of interest statement
Conflict of interest: V. Cottin has received consultancy and lectures fees, and support for travel to medical meetings from Actelion and Roche; has received personal fees for development of educational presentations, consultancy and lectures fees, and support for travel to medical meetings from Boehringer Ingelheim; has received consultancy fees from Bayer and Galapagos; has received personal fees from service on an adjudication committee from Gilead; consultancy fees from GlaxoSmithKline (in 2015) and MSD; has received consultancy and lecture fees from Novartis and Sanofi; is the chair of the data and safety monitoring board (DSMB) of Promedior; and serves on the DSMB of Celgene, all outside the submitted work. Conflict of interest: D. Koschel has received consultancy or speaker fees from AstraZeneca, Boehringer Ingelheim, Grifols, Novartis, Roche/Intermune, Sanofi-Aventis and Teva. Conflict of interest: A. Günther has received research funding from Sanofi, Inventiva and Roche; and consultancy or speaker fees from Boehringer Ingelheim, Roche and Teva. Conflict of interest: C. Albera has received fees from Roche (formerly InterMune), Boehringer Ingelheim, Fibrogen, MSD, GlaxoSmithKline, Bayer and Sanofi for activity as an advisor, consultant or steering committee member; he has also received unrestricted grants from Boehringer Ingelheim. Conflict of interest: A. Azuma has received personal fees from AFT Pharma, Boehringer Ingelheim, InterMune/Roche and Shionogi Co. Conflict of interest: C.M. Sköld has received research funding from Boehringer Ingelheim, Roche and Sandoz; and consultancy or speaker fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, InterMune, Meda, Mundipharma, Novartis, Roche, Sandoz and Vicore Pharma. Conflict of interest: S. Tomassetti has received speaker fees from Boehringer Ingelheim and Roche. Conflict of interest: P. Hormel is an employee of Roche-Genentech and holds shares in that company. Conflict of interest: J.L. Stauffer is an employee of Roche-Genentech and holds shares in that company. Conflict of interest: I. Strombom is an employee of Roche-Genentech and holds shares in that company. Conflict of interest: K-U. Kirchgaessler is an employee of Roche-Genentech and holds shares in that company. Conflict of interest: T.M. Maher has received industry academic research funding from GlaxoSmithKline R&D and UCB Pharma; and consultancy or speaker fees from Apellis, AstraZeneca, aTyr Pharma, Bayer, Biogen Idec, Boehringer Ingelheim, Galapagos, GlaxoSmithKline R&D, ProMetic, Roche (and previously InterMune), Sanumed and UCB Pharma.
Figures
Similar articles
-
A 2-Year Observational Study in Patients Suffering from Idiopathic Pulmonary Fibrosis and Treated with Pirfenidone: A French Ancillary Study of PASSPORT.Respiration. 2019;98(1):19-28. doi: 10.1159/000496735. Epub 2019 Apr 9. Respiration. 2019. PMID: 30965332
-
South-West of England's Experience of the Safety and Tolerability Pirfenidone and Nintedanib for the Treatment of Idiopathic Pulmonary Fibrosis (IPF).Front Pharmacol. 2018 Dec 17;9:1480. doi: 10.3389/fphar.2018.01480. eCollection 2018. Front Pharmacol. 2018. PMID: 30618768 Free PMC article.
-
All-case post-marketing surveillance of 1371 patients treated with pirfenidone for idiopathic pulmonary fibrosis.Respir Investig. 2015 Sep;53(5):232-41. doi: 10.1016/j.resinv.2015.06.001. Epub 2015 Aug 17. Respir Investig. 2015. PMID: 26344613
-
Long-term clinical and real-world experience with pirfenidone in the treatment of idiopathic pulmonary fibrosis.Eur Respir Rev. 2015 Mar;24(135):58-64. doi: 10.1183/09059180.00011514. Eur Respir Rev. 2015. PMID: 25726556 Free PMC article. Review.
-
A safety evaluation of pirfenidone for the treatment of idiopathic pulmonary fibrosis.Expert Opin Drug Saf. 2016 Jul;15(7):975-82. doi: 10.1080/14740338.2016.1187129. Epub 2016 May 26. Expert Opin Drug Saf. 2016. PMID: 27177012 Review.
Cited by
-
Idiopathic pulmonary fibrosis: accurate diagnosis and early treatment.J Bras Pneumol. 2019 Nov 11;45(5):e20190353. doi: 10.1590/1806-3713/e20190353. J Bras Pneumol. 2019. PMID: 31721894 Free PMC article. No abstract available.
-
Managing Fatigue in Patients With Interstitial Lung Disease.Chest. 2020 Nov;158(5):2026-2033. doi: 10.1016/j.chest.2020.04.047. Epub 2020 May 7. Chest. 2020. PMID: 32387518 Free PMC article. Review.
-
Stevens-Johnson syndrome/toxic epidermal necrolysis induced by pirfenidone.Indian J Dermatol Venereol Leprol. 2021 [SEASON];87(4):542-544. doi: 10.25259/IJDVL_61_20. Indian J Dermatol Venereol Leprol. 2021. PMID: 34114417 No abstract available.
-
Real-world safety and effectiveness of pirfenidone and nintedanib in the treatment of idiopathic pulmonary fibrosis: a systematic review and meta-analysis.Eur J Clin Pharmacol. 2024 Oct;80(10):1445-1460. doi: 10.1007/s00228-024-03720-7. Epub 2024 Jul 4. Eur J Clin Pharmacol. 2024. PMID: 38963453
-
Improvement of psoriatic skin lesions following pirfenidone use in patients with fibrotic lung disease.BMJ Case Rep. 2023 Sep 27;16(9):e252591. doi: 10.1136/bcr-2022-252591. BMJ Case Rep. 2023. PMID: 37758659
References
-
- Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011; 183: 431–440. - PubMed
-
- European Medicines Agency. Esbriet (pirfenidone). Summary of Product Characteristics. 2017. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information... Date last accessed: June 1, 2018.
-
- US Food and Drug Administration. Esbriet. Highlights of Prescribing Information 2017. www.accessdata.fda.gov/drugsatfda_docs/label/2017/208780s000lbl.pdf Date last accessed: June 1, 2018.
-
- Pharmaceuticals and Medical Devices Agency Japan. Pirfenidone Review Report 2008. www.pmda.go.jp/files/000153687.pdf Date last accessed: June 4, 2018.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
