Late-pregnancy dysglycemia in obese pregnancies after negative testing for gestational diabetes and risk of future childhood overweight: An interim analysis from a longitudinal mother-child cohort study
- PMID: 30372451
- PMCID: PMC6205663
- DOI: 10.1371/journal.pmed.1002681
Late-pregnancy dysglycemia in obese pregnancies after negative testing for gestational diabetes and risk of future childhood overweight: An interim analysis from a longitudinal mother-child cohort study
Abstract
Background: Maternal pre-conception obesity is a strong risk factor for childhood overweight. However, prenatal mechanisms and their effects in susceptible gestational periods that contribute to this risk are not well understood. We aimed to assess the impact of late-pregnancy dysglycemia in obese pregnancies with negative testing for gestational diabetes mellitus (GDM) on long-term mother-child outcomes.
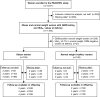
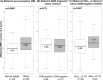
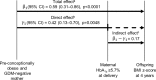
Methods and findings: The prospective cohort study Programming of Enhanced Adiposity Risk in Childhood-Early Screening (PEACHES) (n = 1,671) enrolled obese and normal weight mothers from August 2010 to December 2015 with trimester-specific data on glucose metabolism including GDM status at the end of the second trimester and maternal glycated hemoglobin (HbA1c) at delivery as a marker for late-pregnancy dysglycemia (HbA1c ≥ 5.7% [39 mmol/mol]). We assessed offspring short- and long-term outcomes up to 4 years, and maternal glucose metabolism 3.5 years postpartum. Multivariable linear and log-binomial regression with effects presented as mean increments (Δ) or relative risks (RRs) with 95% confidence intervals (CIs) were used to examine the association between late-pregnancy dysglycemia and outcomes. Linear mixed-effects models were used to study the longitudinal development of offspring body mass index (BMI) z-scores. The contribution of late-pregnancy dysglycemia to the association between maternal pre-conception obesity and offspring BMI was estimated using mediation analysis. In all, 898 mother-child pairs were included in this unplanned interim analysis. Among obese mothers with negative testing for GDM (n = 448), those with late-pregnancy dysglycemia (n = 135, 30.1%) had higher proportions of excessive total gestational weight gain (GWG), excessive third-trimester GWG, and offspring with large-for-gestational-age birth weight than those without. Besides higher birth weight (Δ 192 g, 95% CI 100-284) and cord-blood C-peptide concentration (Δ 0.10 ng/ml, 95% CI 0.02-0.17), offspring of these women had greater weight gain during early childhood (Δ BMI z-score per year 0.18, 95% CI 0.06-0.30, n = 262) and higher BMI z-score at 4 years (Δ 0.58, 95% CI 0.18-0.99, n = 43) than offspring of the obese, GDM-negative mothers with normal HbA1c values at delivery. Late-pregnancy dysglycemia in GDM-negative mothers accounted for about one-quarter of the association of maternal obesity with offspring BMI at age 4 years (n = 151). In contrast, childhood BMI z-scores were not affected by a diagnosis of GDM in obese pregnancies (GDM-positive: 0.58, 95% CI 0.36-0.79, versus GDM-negative: 0.62, 95% CI 0.44-0.79). One mechanism triggering late-pregnancy dysglycemia in obese, GDM-negative mothers was related to excessive third-trimester weight gain (RR 1.72, 95% CI 1.12-2.65). Furthermore, in the maternal population, we found a 4-fold (RR 4.01, 95% CI 1.97-8.17) increased risk of future prediabetes or diabetes if obese, GDM-negative women had a high versus normal HbA1c at delivery (absolute risk: 43.2% versus 10.5%). There is a potential for misclassification bias as the predominantly used GDM test procedure changed over the enrollment period. Further studies are required to validate the findings and elucidate the possible third-trimester factors contributing to future mother-child health status.
Conclusions: Findings from this interim analysis suggest that offspring of obese mothers treated because of a diagnosis of GDM appeared to have a better BMI outcome in childhood than those of obese mothers who-following negative GDM testing-remained untreated in the last trimester and developed dysglycemia. Late-pregnancy dysglycemia related to uncontrolled weight gain may contribute to the development of child overweight and maternal diabetes. Our data suggest that negative GDM testing in obese pregnancies is not an "all-clear signal" and should not lead to reduced attention and risk awareness of physicians and obese women. Effective strategies are needed to maintain third-trimester glycemic and weight gain control among otherwise healthy obese pregnant women.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Obese Nondiabetic Pregnancies and High Maternal Glycated Hemoglobin at Delivery as an Indicator of Offspring and Maternal Postpartum Risks: The Prospective PEACHES Mother-Child Cohort.Clin Chem. 2015 Nov;61(11):1381-90. doi: 10.1373/clinchem.2015.242206. Epub 2015 Aug 11. Clin Chem. 2015. PMID: 26265704
-
Maternal overweight is not an independent risk factor for increased birth weight, leptin and insulin in newborns of gestational diabetic women: observations from the prospective 'EaCH' cohort study.BMC Pregnancy Childbirth. 2018 Jun 20;18(1):250. doi: 10.1186/s12884-018-1889-8. BMC Pregnancy Childbirth. 2018. PMID: 29925339 Free PMC article.
-
HbA1c and Gestational Weight Gain Are Factors that Influence Neonatal Outcome in Mothers with Gestational Diabetes.J Womens Health (Larchmt). 2016 Jun;25(6):579-85. doi: 10.1089/jwh.2015.5432. Epub 2016 Feb 26. J Womens Health (Larchmt). 2016. PMID: 26918922
-
Offspring body size and metabolic profile - effects of lifestyle intervention in obese pregnant women.Dan Med J. 2014 Jul;61(7):B4893. Dan Med J. 2014. PMID: 25123127 Review.
-
Screening and diagnosing gestational diabetes mellitus.Evid Rep Technol Assess (Full Rep). 2012 Oct;(210):1-327. Evid Rep Technol Assess (Full Rep). 2012. PMID: 24423035 Free PMC article. Review.
Cited by
-
Animal Foetal Models of Obesity and Diabetes - From Laboratory to Clinical Settings.Front Endocrinol (Lausanne). 2022 Feb 7;13:785674. doi: 10.3389/fendo.2022.785674. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35197931 Free PMC article. Review.
-
Continuous glucose monitoring in obese pregnant women with no hyperglycemia on glucose tolerance test.PLoS One. 2021 Jun 10;16(6):e0253047. doi: 10.1371/journal.pone.0253047. eCollection 2021. PLoS One. 2021. PMID: 34111215 Free PMC article.
-
Controversies in Gestational Diabetes.touchREV Endocrinol. 2021 Nov;17(2):102-107. doi: 10.17925/EE.2021.17.2.102. Epub 2021 Aug 4. touchREV Endocrinol. 2021. PMID: 35118455 Free PMC article. Review.
-
The Determinants of Adolescent Glycolipid Metabolism Disorder: A Cohort Study.Int J Endocrinol. 2022 Jun 8;2022:6214785. doi: 10.1155/2022/6214785. eCollection 2022. Int J Endocrinol. 2022. PMID: 35719191 Free PMC article.
-
Bidirectional association of neurodevelopment with growth: a prospective cohort study.BMC Pediatr. 2021 Apr 28;21(1):203. doi: 10.1186/s12887-021-02655-7. BMC Pediatr. 2021. PMID: 33910531 Free PMC article.
References
-
- Devlieger R, Benhalima K, Damm P, Van Assche A, Mathieu C, Mahmood T, et al. Maternal obesity in Europe: where do we stand and how to move forward? A scientific paper commissioned by the European Board and College of Obstetrics and Gynaecology (EBCOG). Eur J Obstet Gynecol Reprod Biol. 2016;201:203–8. 10.1016/j.ejogrb.2016.04.005 - DOI - PubMed
-
- Mensink GB, Schienkiewitz A, Haftenberger M, Lampert T, Ziese T, Scheidt-Nave C. [Overweight and obesity in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1)]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56(5–6):786–94. 10.1007/s00103-012-1656-3 - DOI - PubMed
-
- Institut für Qualitätssicherung und Transparenz im Gesundheitswesen. Geburtshilfe (GEBH). Berlin: Institut für Qualitätssicherung und Transparenz im Gesundheitswesen; 2016. [cited 2018 July 27]. Available from: https://iqtig.org/qs-verfahren/gebh/.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

