Probing human sperm metabolism using 13C-magnetic resonance spectroscopy
- PMID: 30395244
- PMCID: PMC6314230
- DOI: 10.1093/molehr/gay046
Probing human sperm metabolism using 13C-magnetic resonance spectroscopy
Abstract
Study question: Can 13C-Magnetic Resonance Spectroscopy (MRS) of selected metabolites provide useful information about human sperm metabolism and how glycolysis or oxidative phosphorylation are used by different sperm populations?
Summary answer: Sperm populations, prepared by density gradient centrifugation (DGC) and incubated with either 13Cu-glucose, 13Cu-fructose or 13C1-pyruvate, showed consistent evidence of metabolism generating principally lactate and more intermittently bicarbonate, and significantly more lactate was produced from 13Cu-glucose by vital or motile sperm recovered from the 40/80% interface compared to those from the pellet, which could not be accounted for by differences in the non-sperm cells present.
What is known already: Previous studies have focused on CO2 or other specific metabolite production by human sperm and there remains considerable debate about whether glycolysis and/or oxidative phosphorylation is the more important pathway for ATP production in sperm.
Study design, size, duration: Sperm populations were prepared by DGC and subjected to 13C-MRS to answer the following questions. (i) Is it possible to detect human sperm metabolism of 13C substrates implicated in energy generation? (ii) What are the kinetics of such reactions? (iii) Do different sperm populations (e.g. '80%' pellet sperm and '40%' interface sperm) utilise substrates in the same way? Semen samples from 97 men were used in these experiments; 52 were used in parallel for aims (i) and (ii) and 45 were used for aim (iii).
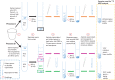
Participants/materials, setting, methods: Sperm populations were prepared from ejaculates of healthy men using a Percoll/Phosphate Buffered Saline (PBS) DGC and then incubated with a range of 13C-labelled substrates (13Cu-glucose, 13Cu-fructose, 13C1-pyruvate, 13C1-butyrate, 13C3-lactate, 13C2,4-D-3-hydroxybutyrate, 13C5-l-glutamate, 13C1,2-glycine or 13Cu-galactose) along with penicillin/streptomycin antibiotic at 37°C for 4 h, 24 h or over 48 h for an estimated rate constant. Sperm concentration, vitality and motility were measured and, for a subset of experiments, non-sperm cell concentration was determined. A 9.4 T magnetic resonance spectrometer was used to acquire 1D 13C, inverse gated 1H decoupled, MRS spectra. Spectrum processing was carried out using spectrometer software and Matlab scripts to determine peak integrals for each spectrum.
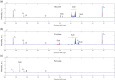
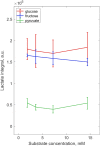
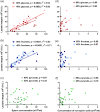
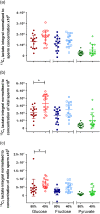
Main results and the role of chance: 13Cu-glucose, 13Cu-fructose and 13C1-pyruvate were consistently converted into lactate and, to a lesser extent, bicarbonate. There was a significant correlation between sperm concentration and lactate peak size for 13Cu-glucose and 13Cu-fructose, which was not observed for 13C1-pyruvate. The lactate peak did not correlate with the non-sperm cell concentration up to 6.9 × 106/ml. The concentration of 13Cu-glucose, 13Cu-fructose or 13C1-pyruvate (1.8, 3.6, 7.2 or 14.4 mM) had no influence on the size of the observed lactate peak over a 4 h incubation. The rate of conversion of 13C1-pyruvate to lactate was approximately three times faster than for 13Cu-glucose or 13Cu-fructose which were not significantly different from each other. After incubating for 4 h, the utilisation of 13Cu-glucose, 13Cu-fructose or 13C1-pyruvate by sperm from the '40%' interface of the DGC was no different from those from the pellet when normalised to total sperm concentration. However, after normalising by either the vital or motile sperm concentration, there was a significant increase in conversion of 13Cu-glucose to lactate by '40%' interface sperm compared to pellet sperm (Vital = 3.3 ± 0.30 × 106 vs 2.0 ± 0.21 × 106; P = 0.0049; Motile = 7.0 ± 0.75 × 106 vs 4.8 ± 0.13 × 106; P = 0.0032. Mann-Whitney test P < 0.0055 taken as statistically significant). No significant differences were observed for 13Cu-fructose or 13C1-pyruvate.
Large scale data: Not applicable.
Limitations, reasons for caution: Only 13C labelled metabolites that accumulate to a sufficiently high concentration can be observed by 13C MRS. For this reason, intermediary molecules in the metabolic chain are difficult to observe without trapping the molecule at a particular step using inhibitors. Non-sperm cell concentration was typical of the general population and no link was found between these cells and the magnitude of the 13C-lactate peak. However, it is possible that higher concentrations than the maximum observed (6.9 × 106/ml) may contribute to exogenous substrate metabolism in other experiments.
Wider implications of the findings: 13C-MRS can provide information on the underlying metabolism of multiple pathways in live sperm. Dysfunction in sperm metabolism, as a result of either impaired enzymes of lack of metabolisable substrate, could be detected in sperm by a non-destructive assay, potentially offering new treatment options to improve overall sperm quality and outcomes for reproduction.
Study funding and competing interests: This work was supported by the Medical Research Council Grant MR/M010473/1. The authors declare no conflicts of interest.
Figures
Similar articles
-
1H Magnetic Resonance Spectroscopy of live human sperm.Mol Hum Reprod. 2017 Jul 1;23(7):441-451. doi: 10.1093/molehr/gax025. Mol Hum Reprod. 2017. PMID: 28431025 Free PMC article.
-
NMR spectroscopy of live human asthenozoospermic and normozoospermic sperm metabolism.Reprod Fertil. 2022 Mar 21;3(2):77-89. doi: 10.1530/RAF-21-0101. eCollection 2022 Apr 1. Reprod Fertil. 2022. PMID: 35514541 Free PMC article.
-
Analysis of metabolic flux in felid spermatozoa using metabolomics and 13C-based fluxomics†.Biol Reprod. 2019 May 1;100(5):1261-1274. doi: 10.1093/biolre/ioz010. Biol Reprod. 2019. PMID: 30715249
-
Probing carbohydrate metabolism using hyperpolarized 13 C-labeled molecules.NMR Biomed. 2019 Oct;32(10):e4018. doi: 10.1002/nbm.4018. Epub 2018 Nov 26. NMR Biomed. 2019. PMID: 30474153 Free PMC article. Review.
-
Hyperpolarized 13C-labeled bicarbonate (H13CO3-) for in vivo pH measurement with 13C magnetic resonance spectroscopy.2010 Jan 25 [updated 2010 Apr 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2010 Jan 25 [updated 2010 Apr 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641986 Free Books & Documents. Review.
Cited by
-
Centrifugation Force and Time Alter CASA Parameters and Oxidative Status of Cryopreserved Stallion Sperm.Biology (Basel). 2020 Jan 27;9(2):22. doi: 10.3390/biology9020022. Biology (Basel). 2020. PMID: 32012799 Free PMC article.
-
Capacitation induces changes in metabolic pathways supporting motility of epididymal and ejaculated sperm.Front Cell Dev Biol. 2023 Jun 27;11:1160154. doi: 10.3389/fcell.2023.1160154. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37440924 Free PMC article.
-
Efficacy and mechanism of lipoic acid in the treatment of reproductive injury caused by perfluorooctanoic acid.Exp Ther Med. 2023 Jan 30;25(3):116. doi: 10.3892/etm.2023.11815. eCollection 2023 Mar. Exp Ther Med. 2023. PMID: 36815965 Free PMC article.
-
The utility of nuclear magnetic resonance spectroscopy in assisted reproduction.Open Biol. 2020 Nov;10(11):200092. doi: 10.1098/rsob.200092. Epub 2020 Nov 4. Open Biol. 2020. PMID: 33142083 Free PMC article. Review.
-
Sperm Metabolomics through Nuclear Magnetic Resonance Spectroscopy.Animals (Basel). 2021 Jun 3;11(6):1669. doi: 10.3390/ani11061669. Animals (Basel). 2021. PMID: 34205204 Free PMC article. Review.
References
-
- Aitken RJ, Buckingham DW, Carreras A, Irvine DS. Superoxide dismutase in human sperm suspensions: relationship with cellular composition, oxidative stress, and sperm function. Free Radical Bio Med 1996;4:495–504. - PubMed
-
- Aitken RJ, West KM. Analysis of the relationship between reactive oxygen species production and leucocyte infiltration in fractions of human semen separated on Percoll gradients. Int J Androl 1990;6:433–451. - PubMed
-
- Amaral A, Paiva C, Attardo Parrinello C, Estanyol JM, Ballesca JL, Ramalho-Santos J, Oliva R. Identification of proteins involved in human sperm motility using high-throughput differential proteomics. J Proteome Res 2014;12:5670–5684. - PubMed
-
- Andrade-Rocha FT. Seminal fructose levels in male infertility: relationship with sperm characteristics. Int Urol Nephrol 1999;1:107–111. - PubMed
-
- Angulo C, Rauch MC, Droppelmann A, Reyes AM, Slebe JC, Delgado-Lopez F, Guaiquil VH, Vera JC. Concha, II. Hexose transporter expression and function in mammalian spermatozoa: cellular localization and transport of hexoses and vitamin C. J Cell Biochem 1998;2:189–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

