Investigation of allele-specific expression of genes involved in adipogenesis and lipid metabolism suggests complex regulatory mechanisms of PPARGC1A expression in porcine fat tissues
- PMID: 30497374
- PMCID: PMC6267897
- DOI: 10.1186/s12863-018-0696-6
Investigation of allele-specific expression of genes involved in adipogenesis and lipid metabolism suggests complex regulatory mechanisms of PPARGC1A expression in porcine fat tissues
Abstract
Background: The expression of genes involved in regulating adipogenesis and lipid metabolism may affect economically important fatness traits in pigs. Allele-specific expression (ASE) reflects imbalance between allelic transcript levels and can be used to identify underlying cis-regulatory elements. ASE has not yet been intensively studied in pigs. The aim of this investigation was to analyze the differential allelic expression of four genes, PPARA, PPARG, SREBF1, and PPARGC1A, which are involved in the regulation of fat deposition in porcine subcutaneous and visceral fat and longissimus dorsi muscle.
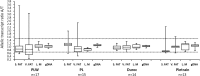
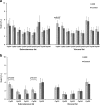
Results: Quantification of allelic proportions by pyrosequencing revealed that both alleles of PPARG and SREBF1 are expressed at similar levels. PPARGC1A showed the greatest ASE imbalance in fat deposits in Polish Large White (PLW), Polish Landrace and Pietrain pigs; and PPARA in PLW pigs. Significant deviations of mean PPARGC1A allelic transcript ratio between cDNA and genomic DNA were detected in all tissues, with the most pronounced difference (p < 0.001) in visceral fat of PLW pigs. To search for potential cis-regulatory elements affecting ASE in the PPARGC1A gene we analyzed the effects of four SNPs (rs337351686, rs340650517, rs336405906 and rs345224049) in the promoter region, but none were associated with ASE in the breeds studied. DNA methylation analysis revealed significant CpG methylation differences between samples showing balanced (allelic transcript ratio ≈1) and imbalanced allelic expression for CpG site at the genomic position in chromosome 8 (SSC8): 18527678 in visceral fat (p = 0.017) and two CpG sites (SSC8:18525215, p = 0.030; SSC8:18525237, p = 0.031) in subcutaneous fat.
Conclusions: Our analysis of differential allelic expression suggests that PPARGC1A is subjected to cis-regulation in porcine fat tissues. Further studies are necessary to identify other regulatory elements localized outside the PPARGC1A proximal promoter region.
Keywords: Allele-specific expression; CpG methylation; Fat; Muscle; PPARA; PPARG; PPARGC1A; Pig; SREBF1.
Conflict of interest statement
Ethics approval and consent to participate
All animal procedures performed for the purpose of the study were approved by the Local Ethical Commission on Experiments on Animals at the Poznan University of Life Sciences, Poznan, Poland (no. 57/2012).
Consent for publication
Not Applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Analysis of allele-specific expression of seven candidate genes involved in lipid metabolism in pig skeletal muscle and fat tissues reveals allelic imbalance of ACACA, LEP, SCD, and TNF.J Appl Genet. 2019 Feb;60(1):97-101. doi: 10.1007/s13353-019-00485-z. Epub 2019 Jan 26. J Appl Genet. 2019. PMID: 30684136 Free PMC article.
-
Polymorphism in 3' untranslated region of the pig PPARA gene influences its transcript level and is associated with adipose tissue accumulation.J Anim Sci. 2014 Jun;92(6):2363-71. doi: 10.2527/jas.2013-7509. Epub 2014 Mar 26. J Anim Sci. 2014. PMID: 24671595
-
Expression of genes involved in adipogenesis and lipid metabolism in subcutaneous adipose tissue and longissimus muscle in low-marbled Pirenaica beef cattle.Animal. 2016 Dec;10(12):2018-2026. doi: 10.1017/S175173111600118X. Epub 2016 Jun 24. Animal. 2016. PMID: 27339509
-
Molecular Regulation of Lipogenesis, Adipogenesis and Fat Deposition in Chicken.Genes (Basel). 2021 Mar 13;12(3):414. doi: 10.3390/genes12030414. Genes (Basel). 2021. PMID: 33805667 Free PMC article. Review.
-
Allele-specific DNA methylation: beyond imprinting.Hum Mol Genet. 2010 Oct 15;19(R2):R210-20. doi: 10.1093/hmg/ddq376. Epub 2010 Sep 20. Hum Mol Genet. 2010. PMID: 20855472 Free PMC article. Review.
Cited by
-
Genomic analyses provide insights into breed-of-origin effects from purebreds on three-way crossbred pigs.PeerJ. 2019 Nov 11;7:e8009. doi: 10.7717/peerj.8009. eCollection 2019. PeerJ. 2019. PMID: 31737448 Free PMC article.
-
Analysis of allele-specific expression of seven candidate genes involved in lipid metabolism in pig skeletal muscle and fat tissues reveals allelic imbalance of ACACA, LEP, SCD, and TNF.J Appl Genet. 2019 Feb;60(1):97-101. doi: 10.1007/s13353-019-00485-z. Epub 2019 Jan 26. J Appl Genet. 2019. PMID: 30684136 Free PMC article.
-
The importance of the nuclear positioning of the PPARG gene for its expression during porcine in vitro adipogenesis.Chromosome Res. 2019 Sep;27(3):271-284. doi: 10.1007/s10577-019-09604-2. Epub 2019 Jan 17. Chromosome Res. 2019. PMID: 30656515 Free PMC article.
-
Analysis of long intergenic non-coding RNAs transcriptomic profiling in skeletal muscle growth during porcine embryonic development.Sci Rep. 2021 Jul 27;11(1):15240. doi: 10.1038/s41598-021-94014-w. Sci Rep. 2021. PMID: 34315913 Free PMC article.
-
Transcriptome study digs out BMP2 involved in adipogenesis in sheep tails.BMC Genomics. 2022 Jun 21;23(1):457. doi: 10.1186/s12864-022-08657-8. BMC Genomics. 2022. PMID: 35725366 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

