Quantitative Proteomic Analysis Identifies MAPK15 as a Potential Regulator of Radioresistance in Nasopharyngeal Carcinoma Cells
- PMID: 30524968
- PMCID: PMC6262088
- DOI: 10.3389/fonc.2018.00548
Quantitative Proteomic Analysis Identifies MAPK15 as a Potential Regulator of Radioresistance in Nasopharyngeal Carcinoma Cells
Abstract
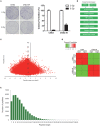
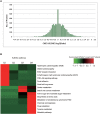
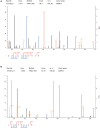
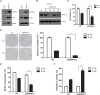
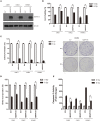
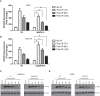
Since resistance to radiotherapy remains refractory for the clinical management of nasopharyngeal cancer (NPC), further understanding the mechanisms of radioresistance is necessary in order to develop more effective NPC treatment and improve prognosis. In this study, an integrated quantitative proteomic approach involving tandem mass tag labeling and liquid chromatograph-mass spectrometer was used to identify proteins potentially responsible for the radioresistance of NPC. The differential radiosensitivity in NPC model cells was examined through clonogenic survival assay, CCK-8 viability assay, and BrdU incorporation analysis. Apoptosis of NPC cells after exposure to irradiation was detected using caspase-3 colorimetric assay. Intracellular reactive oxygen species (ROS) was detected by a dichlorofluorescin diacetate fluorescent probe. In total, 5,946 protein groups were identified, among which 5,185 proteins were quantified. KEGG pathway analysis and protein-protein interaction enrichment analysis revealed robust activation of multiple biological processes/pathways in radioresistant CNE2-IR cells. Knockdown of MAPK15, one up-regulated protein kinase in CNE2-IR cells, significantly impaired clonogenic survival, decreased cell viability and increased cell apoptosis following exposure to irradiation, while over-expression of MAPK15 promoted cell survival, induced radioresistance and reduced apoptosis in NPC cell lines CNE1, CNE2, and HONE1. MAPK15 might regulate radioresistance through attenuating ROS accumulation and promoting DNA damage repair after exposure to irradiation in NPC cells. Quantitative proteomic analysis revealed enormous metabolic processes/signaling networks were potentially involved in the radioresistance of NPC cells. MAPK15 might be a novel potential regulator of radioresistance in NPC cells, and _targeting MAPK15 might be useful in sensitizing NPC cells to radiotherapy.
Keywords: MAPK15; nasopharyngeal carcinoma; quantitative proteomics; radioresistance; radiosensitivity.
Figures

Similar articles
-
Identification of heat shock protein 27 as a radioresistance-related protein in nasopharyngeal carcinoma cells.J Cancer Res Clin Oncol. 2012 Dec;138(12):2117-25. doi: 10.1007/s00432-012-1293-0. Epub 2012 Jul 31. J Cancer Res Clin Oncol. 2012. PMID: 22847231
-
Quantitative proteome analysis identifies MAP2K6 as potential regulator of LIFR-induced radioresistance in nasopharyngeal carcinoma cells.Biochem Biophys Res Commun. 2018 Oct 20;505(1):274-281. doi: 10.1016/j.bbrc.2018.09.020. Epub 2018 Sep 21. Biochem Biophys Res Commun. 2018. PMID: 30245131
-
Quantitative Tyrosine Phosphoproteomic Analysis of Resistance to Radiotherapy in Nasopharyngeal Carcinoma Cells.Cancer Manag Res. 2020 Dec 9;12:12667-12678. doi: 10.2147/CMAR.S260028. eCollection 2020. Cancer Manag Res. 2020. PMID: 33328764 Free PMC article.
-
Multiple Mechanisms Involving in Radioresistance of Nasopharyngeal Carcinoma.J Cancer. 2020 Apr 25;11(14):4193-4204. doi: 10.7150/jca.39354. eCollection 2020. J Cancer. 2020. PMID: 32368302 Free PMC article. Review.
-
Biomarkers for enhancing the radiosensitivity of nasopharyngeal carcinoma.Cancer Biol Med. 2015 Mar;12(1):23-32. doi: 10.7497/j.issn.2095-3941.2014.0015. Cancer Biol Med. 2015. PMID: 25859408 Free PMC article. Review.
Cited by
-
Hypofractionated Radiation Versus Conventional Fractionated Radiation: A Network Analysis.J Lasers Med Sci. 2022 Sep 23;13:e39. doi: 10.34172/jlms.2022.39. eCollection 2022. J Lasers Med Sci. 2022. PMID: 36743138 Free PMC article.
-
Biomarkers of tumor invasiveness in proteomics (Review).Int J Oncol. 2020 Aug;57(2):409-432. doi: 10.3892/ijo.2020.5075. Epub 2020 May 28. Int J Oncol. 2020. PMID: 32468071 Free PMC article.
-
Proteomic approaches to investigate gammaherpesvirus biology and associated tumorigenesis.Adv Virus Res. 2021;109:201-254. doi: 10.1016/bs.aivir.2020.10.001. Epub 2020 Nov 9. Adv Virus Res. 2021. PMID: 33934828 Free PMC article. Review.
-
Growth and differentiation factor 15 regulates PD-L1 expression in glioblastoma.Cancer Manag Res. 2019 Apr 2;11:2653-2661. doi: 10.2147/CMAR.S192095. eCollection 2019. Cancer Manag Res. 2019. PMID: 31114328 Free PMC article.
-
Quantitative Proteomic Profiling Identifies SOX8 as Novel Regulator of Drug Resistance in Gestational Trophoblastic Neoplasia.Front Oncol. 2020 Apr 28;10:557. doi: 10.3389/fonc.2020.00557. eCollection 2020. Front Oncol. 2020. PMID: 32411596 Free PMC article.
References
-
- Zhou Q, He Y, Zhao Y, Wang Y, Kuang W, Shen L. A study of 358 cases of locally advanced nasopharyngeal carcinoma receiving intensity-modulated radiation therapy: improving the seventh edition of the american joint committee on cancer T-staging system. Biomed Res Int. (2017) 2017:1419676. 10.1155/2017/1419676 - DOI - PMC - PubMed
-
- Hu Y, E H, Yu X, Li F, Zeng L, Lu Q, et al. . Correlation of quantitative parameters of magnetic resonance perfusion-weighted imaging with vascular endothelial growth factor, microvessel density and hypoxia-inducible factor-1alpha in nasopharyngeal carcinoma: Evaluation on radiosensitivity study. Clin Otolaryngol. (2018) 43:425–33. 10.1111/coa.12982 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

