Internal enhancement of DNA damage by a novel bispecific antibody-drug conjugate-like therapeutics via blockage of mTOR and PD-L1 signal pathways in pancreatic cancer
- PMID: 30681288
- PMCID: PMC6382721
- DOI: 10.1002/cam4.1974
Internal enhancement of DNA damage by a novel bispecific antibody-drug conjugate-like therapeutics via blockage of mTOR and PD-L1 signal pathways in pancreatic cancer
Abstract
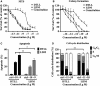
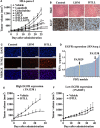
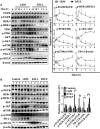
Pancreatic ductal adenocarcinoma (PDAC) is a refractory malignant tumor with poor prognosis, limited chemotherapeutic efficacy, and only about 5% of 5-year survival rate. We generated a dual-_targeting ligand-based lidamycin (DTLL) to investigate its efficacy against pancreatic cancer after preparing its precursor, DTLP. DTLP was shown specifically binding to EGFR and HER2 on cell surface, followed by endocytosis into cytoplasm of pancreatic cancer cells. DTLL significantly promoted apoptosis and cell cycle arrest at G2/M stages and inhibited cell proliferation. Pancreatic tumors of either MIA-paca-2 cell line-derived (CDX) or patient-derived xenograft (PDX) mouse models were significantly regressed in response to DTLL. It suggested that DTLL might be a highly potent bispecific antibody-drug conjugate (ADC)-like agent for pancreatic cancer therapy. LDM is known to function as an antitumor cytotoxic agent by its induction of DNA damage in cancer cells, therefore, DTLL, as its derivative, also showed similar cytotoxicity. However, we found that DTLL might reverse the AKT/mTOR feedback activation induced by LDM at the first time. The results from both in vitro and in vivo experiments suggested that DTLL enhanced DNA damage via EGFR/HER2-dependent blockage of PI3K/AKT/mTOR and PD-L1 signaling pathways in cancer cells, leading to the inhibition of cell proliferation and immunosurveillance escape from pancreatic tumor. Our studies on DTLL functional characterization revealed its novel mechanisms on internal enhancement of DNA damage and implied that DTLL might provide a promising _targeted therapeutic strategy for pancreatic cancer.
Keywords: ADC-like therapeutic agent; EGFR; HER2; bispecificity; pancreatic ductal adenocarcinoma.
© 2019 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures
Similar articles
-
An EGFR/HER2-_targeted conjugate sensitizes gemcitabine-sensitive and resistant pancreatic cancer through different SMAD4-mediated mechanisms.Nat Commun. 2022 Sep 20;13(1):5506. doi: 10.1038/s41467-022-33037-x. Nat Commun. 2022. PMID: 36127339 Free PMC article.
-
A Macropinocytosis-Intensifying Albumin Domain-Based scFv Antibody and Its Conjugate Directed against K-Ras Mutant Pancreatic Cancer.Mol Pharm. 2018 Jun 4;15(6):2403-2412. doi: 10.1021/acs.molpharmaceut.8b00234. Epub 2018 May 23. Mol Pharm. 2018. PMID: 29757658
-
mTOR inhibition induces EGFR feedback activation in association with its resistance to human pancreatic cancer.Int J Mol Sci. 2015 Feb 3;16(2):3267-82. doi: 10.3390/ijms16023267. Int J Mol Sci. 2015. PMID: 25654224 Free PMC article.
-
_targeting the Akt/PI3K Signaling Pathway as a Potential Therapeutic Strategy for the Treatment of Pancreatic Cancer.Curr Med Chem. 2017;24(13):1321-1331. doi: 10.2174/0929867324666170206142658. Curr Med Chem. 2017. PMID: 28176634 Review.
-
Epidermal Growth Factor Receptor and Its Role in Pancreatic Cancer Treatment Mediated by Nanoparticles.Int J Nanomedicine. 2019 Dec 9;14:9693-9706. doi: 10.2147/IJN.S226628. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31849462 Free PMC article. Review.
Cited by
-
Research progress on novel antibody drug conjugates in cancer therapy.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024 Feb 28;49(2):296-304. doi: 10.11817/j.issn.1672-7347.2024.230418. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38755726 Free PMC article. Review. Chinese, English.
-
An EGFR/HER2-_targeted conjugate sensitizes gemcitabine-sensitive and resistant pancreatic cancer through different SMAD4-mediated mechanisms.Nat Commun. 2022 Sep 20;13(1):5506. doi: 10.1038/s41467-022-33037-x. Nat Commun. 2022. PMID: 36127339 Free PMC article.
-
Anticancer Activity of Urease Mimetic Cobalt (III) Complexes on A549-Lung Cancer Cells: _targeting the Acidic Microenvironment.Pharmaceutics. 2022 Jan 17;14(1):211. doi: 10.3390/pharmaceutics14010211. Pharmaceutics. 2022. PMID: 35057107 Free PMC article.
-
PD-L1 upregulation accompanied with epithelial-mesenchymal transition attenuates sensitivity to ATR inhibition in p53 mutant pancreatic cancer cells.Med Oncol. 2020 Apr 10;37(5):47. doi: 10.1007/s12032-020-01372-y. Med Oncol. 2020. PMID: 32277292
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7‐30. - PubMed
-
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first‐line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403‐2413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

