Natural Killer Cells Degenerate Intact Sensory Afferents following Nerve Injury
- PMID: 30712871
- PMCID: PMC6418410
- DOI: 10.1016/j.cell.2018.12.022
Natural Killer Cells Degenerate Intact Sensory Afferents following Nerve Injury
Abstract
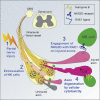
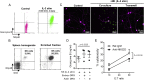
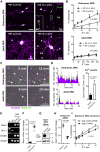
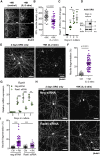
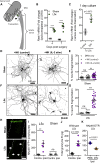
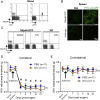
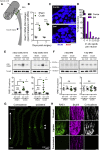
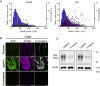
Sensory axons degenerate following separation from their cell body, but partial injury to peripheral nerves may leave the integrity of damaged axons preserved. We show that an endogenous ligand for the natural killer (NK) cell receptor NKG2D, Retinoic Acid Early 1 (RAE1), is re-expressed in adult dorsal root ganglion neurons following peripheral nerve injury, triggering selective degeneration of injured axons. Infiltration of cytotoxic NK cells into the sciatic nerve by extravasation occurs within 3 days following crush injury. Using a combination of genetic cell ablation and cytokine-antibody complex stimulation, we show that NK cell function correlates with loss of sensation due to degeneration of injured afferents and reduced incidence of post-injury hypersensitivity. This neuro-immune mechanism of selective NK cell-mediated degeneration of damaged but intact sensory axons complements Wallerian degeneration and suggests the therapeutic potential of modulating NK cell function to resolve painful neuropathy through the clearance of partially damaged nerves.
Keywords: Wallerian degeneration; autoimmunity; dorsal root ganglia; innate immunity; natural cytotoxicity; neurodegeneration; neuroimmune; neuropathic pain; peripheral neuropathy; sciatic nerve crush.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

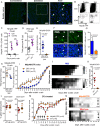
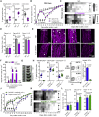
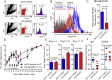
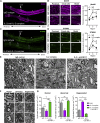

Comment in
-
Pain killers.Nat Rev Immunol. 2019 Mar;19(3):136-137. doi: 10.1038/s41577-019-0137-4. Nat Rev Immunol. 2019. PMID: 30733595 No abstract available.
Similar articles
-
NKG2D-retinoic acid early inducible-1 recognition between natural killer cells and Kupffer cells in a novel murine natural killer cell-dependent fulminant hepatitis.Hepatology. 2009 Mar;49(3):940-9. doi: 10.1002/hep.22725. Hepatology. 2009. PMID: 19177594
-
Distinct calcitonin gene-related peptide expression pattern in primary afferents contribute to different neuropathic symptoms following chronic constriction or crush injuries to the rat sciatic nerve.Mol Pain. 2016 Jan-Dec;12:1744806916681566. doi: 10.1177/1744806916681566. Mol Pain. 2016. PMID: 28256957 Free PMC article.
-
Activating receptor NKG2D _targets RAE-1-expressing allogeneic neural precursor cells in a viral model of multiple sclerosis.Stem Cells. 2014 Oct;32(10):2690-701. doi: 10.1002/stem.1760. Stem Cells. 2014. PMID: 24898518 Free PMC article.
-
Cytotoxic Immunity in Peripheral Nerve Injury and Pain.Front Neurosci. 2020 Feb 21;14:142. doi: 10.3389/fnins.2020.00142. eCollection 2020. Front Neurosci. 2020. PMID: 32153361 Free PMC article. Review.
-
The assessment of neuronal plasticity following sciatic nerve injuries in rats using electron microscopy and stereological methods.J Chem Neuroanat. 2024 Mar;136:102396. doi: 10.1016/j.jchemneu.2024.102396. Epub 2024 Feb 6. J Chem Neuroanat. 2024. PMID: 38331230 Review.
Cited by
-
Inducible knockout of Clec16a in mice results in sensory neurodegeneration.Sci Rep. 2021 Apr 29;11(1):9319. doi: 10.1038/s41598-021-88895-0. Sci Rep. 2021. PMID: 33927318 Free PMC article.
-
The Genetics of Neuropathic Pain from Model Organisms to Clinical Application.Neuron. 2019 Nov 20;104(4):637-653. doi: 10.1016/j.neuron.2019.09.018. Neuron. 2019. PMID: 31751545 Free PMC article. Review.
-
Mechanisms, Characteristics, and Treatment of Neuropathic Pain and Peripheral Neuropathy Associated with Dinutuximab in Neuroblastoma Patients.Int J Mol Sci. 2021 Nov 23;22(23):12648. doi: 10.3390/ijms222312648. Int J Mol Sci. 2021. PMID: 34884452 Free PMC article. Review.
-
Natural killer cells modulate motor neuron-immune cell cross talk in models of Amyotrophic Lateral Sclerosis.Nat Commun. 2020 Apr 14;11(1):1773. doi: 10.1038/s41467-020-15644-8. Nat Commun. 2020. PMID: 32286313 Free PMC article.
-
NK cells associate with ALS in a sex- and age-dependent manner.JCI Insight. 2021 Jun 8;6(11):e147129. doi: 10.1172/jci.insight.147129. JCI Insight. 2021. PMID: 33974561 Free PMC article.
References
-
- Backström E., Chambers B.J., Kristensson K., Ljunggren H.G. Direct NK cell-mediated lysis of syngenic dorsal root ganglia neurons in vitro. J. Immunol. 2000;165:4895–4900. - PubMed
-
- Backström E., Chambers B.J., Ho E.L., Naidenko O.V., Mariotti R., Fremont D.H., Yokoyama W.M., Kristensson K., Ljunggren H.G. Natural killer cell-mediated lysis of dorsal root ganglia neurons via RAE1/NKG2D interactions. Eur. J. Immunol. 2003;33:92–100. - PubMed
-
- Backström E., Ljunggren H.G., Kristensson K. NK cell-mediated destruction of influenza A virus-infected peripheral but not central neurones. Scand. J. Immunol. 2007;65:353–361. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

