Gcn5 histone acetyltransferase is present in the mitoplasts
- PMID: 30777878
- PMCID: PMC6398455
- DOI: 10.1242/bio.041244
Gcn5 histone acetyltransferase is present in the mitoplasts
Abstract
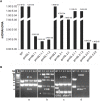
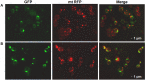
In Saccharomyces cerevisiae the Lysine-acetyltransferase Gcn5 (KAT2) is part of the SAGA complex and is responsible for histone acetylation widely or at specific lysines. In this paper we report that G CN5 deletion differently affects the growth of two strains. The defective mitochondrial phenotype is related to a marked decrease in mtDNA content, which also involves the deletion of specific regions of the molecule. We also show that in wild-type mitochondria the Gcn5 protein is present in the mitoplasts, suggesting a new mitochondrial function independent from the SAGA complex and possibly a new function for this protein connecting epigenetics and metabolism.
Keywords: Lysine-acetyltransferase Gcn5; Mitochondria; Mitochondrial DNA; Respiration; Yeast.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures




Similar articles
-
SAGA complex and Gcn5 are necessary for respiration in budding yeast.Biochim Biophys Acta. 2016 Dec;1863(12):3160-3168. doi: 10.1016/j.bbamcr.2016.10.002. Epub 2016 Oct 11. Biochim Biophys Acta. 2016. PMID: 27741413
-
The SAGA complex in the rice pathogen Fusarium fujikuroi: structure and functional characterization.Mol Microbiol. 2016 Dec;102(6):951-974. doi: 10.1111/mmi.13528. Epub 2016 Sep 30. Mol Microbiol. 2016. PMID: 27642009
-
A conserved central region of yeast Ada2 regulates the histone acetyltransferase activity of Gcn5 and interacts with phospholipids.J Mol Biol. 2008 Dec 26;384(4):743-55. doi: 10.1016/j.jmb.2008.09.088. Epub 2008 Oct 11. J Mol Biol. 2008. PMID: 18950642
-
Adenovirus E1A requires the yeast SAGA histone acetyltransferase complex and associates with SAGA components Gcn5 and Tra1.Oncogene. 2002 Feb 21;21(9):1411-22. doi: 10.1038/sj.onc.1205201. Oncogene. 2002. PMID: 11857084
-
Epigenetic Factors and Mitochondrial Biology in Yeast: A New Paradigm for the Study of Cancer Metabolism?Front Pharmacol. 2018 Nov 21;9:1349. doi: 10.3389/fphar.2018.01349. eCollection 2018. Front Pharmacol. 2018. PMID: 30524288 Free PMC article. Review.
Cited by
-
Catalysis by protein acetyltransferase Gcn5.Biochim Biophys Acta Gene Regul Mech. 2021 Feb;1864(2):194627. doi: 10.1016/j.bbagrm.2020.194627. Epub 2020 Aug 22. Biochim Biophys Acta Gene Regul Mech. 2021. PMID: 32841743 Free PMC article. Review.
-
_targeting the SAGA and ATAC Transcriptional Coactivator Complexes in MYC-Driven Cancers.Cancer Res. 2020 May 15;80(10):1905-1911. doi: 10.1158/0008-5472.CAN-19-3652. Epub 2020 Feb 24. Cancer Res. 2020. PMID: 32094302 Free PMC article. Review.
-
Nuclear metabolism and the regulation of the epigenome.Nat Metab. 2020 Nov;2(11):1190-1203. doi: 10.1038/s42255-020-00285-4. Epub 2020 Oct 12. Nat Metab. 2020. PMID: 33046909 Review.
-
Two genomes, one cell: Mitochondrial-nuclear coordination via epigenetic pathways.Mol Metab. 2020 Aug;38:100942. doi: 10.1016/j.molmet.2020.01.006. Epub 2020 Feb 15. Mol Metab. 2020. PMID: 32217072 Free PMC article. Review.
-
GCN5 acetyltransferase in cellular energetic and metabolic processes.Biochim Biophys Acta Gene Regul Mech. 2021 Feb;1864(2):194626. doi: 10.1016/j.bbagrm.2020.194626. Epub 2020 Aug 19. Biochim Biophys Acta Gene Regul Mech. 2021. PMID: 32827753 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

