Antibody-Drug Conjugates: Possibilities and Challenges
- PMID: 30800238
- PMCID: PMC6359697
Antibody-Drug Conjugates: Possibilities and Challenges
Abstract
The design of Antibody Drug Conjugates (ADCs) as efficient _targeting agents for tumor cell is still in its infancy for clinical applications. This approach incorporates the antibody specificity and cell killing activity of chemically conjugated cytotoxic agents. Antibody in ADC structure acts as a _targeting agent and a nanoscale carrier to deliver a therapeutic dose of cytotoxic cargo into desired tumor cells. Early ADCs encountered major obstacles including, low blood residency time, low penetration capacity to tumor microenvironment, low payload potency, immunogenicity, unusual off-_target toxicity, drug resistance, and the lack of stable linkage in blood circulation. Although extensive studies have been conducted to overcome these issues, the ADCs based therapies are still far from having high-efficient clinical outcomes. This review outlines the key characteristics of ADCs including tumor marker, antibody, cytotoxic payload, and linkage strategy with a focus on technical improvement and some future trends in the pipeline.
Keywords: Antibody-Drug; Cancer therapy; Cytotoxic drugs; Monoclonal antibodies; Nanomedicine.
Conflict of interest statement
Conflict of Interest The authors declare that they have no competing interests.
Figures



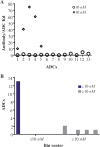

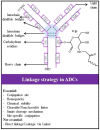
Similar articles
-
Antibody drug conjugates in gastrointestinal cancer: From lab to clinical development.J Control Release. 2021 Dec 10;340:1-34. doi: 10.1016/j.jconrel.2021.10.006. Epub 2021 Oct 19. J Control Release. 2021. PMID: 34673122 Review.
-
Antibody-drug conjugates and bispecific antibodies _targeting cancers: applications of click chemistry.Arch Pharm Res. 2023 Mar;46(3):131-148. doi: 10.1007/s12272-023-01433-6. Epub 2023 Mar 6. Arch Pharm Res. 2023. PMID: 36877356 Review.
-
Antibody-drug conjugates: Resurgent anticancer agents with multi-_targeted therapeutic potential.Pharmacol Ther. 2022 Aug;236:108106. doi: 10.1016/j.pharmthera.2021.108106. Epub 2022 Jan 4. Pharmacol Ther. 2022. PMID: 34990642 Review.
-
In vitro and in vivo evaluation of cysteine and site specific conjugated herceptin antibody-drug conjugates.PLoS One. 2014 Jan 14;9(1):e83865. doi: 10.1371/journal.pone.0083865. eCollection 2014. PLoS One. 2014. PMID: 24454709 Free PMC article.
-
Advances in _targeted Therapy of Breast Cancer with Antibody-Drug Conjugate.Pharmaceutics. 2023 Apr 14;15(4):1242. doi: 10.3390/pharmaceutics15041242. Pharmaceutics. 2023. PMID: 37111727 Free PMC article. Review.
Cited by
-
BCMA-_targeted immunotherapy for multiple myeloma.J Hematol Oncol. 2020 Sep 17;13(1):125. doi: 10.1186/s13045-020-00962-7. J Hematol Oncol. 2020. PMID: 32943087 Free PMC article. Review.
-
B7 homologue 3 as a prognostic biomarker and potential therapeutic _target in gastrointestinal tumors.World J Gastrointest Oncol. 2021 Aug 15;13(8):799-821. doi: 10.4251/wjgo.v13.i8.799. World J Gastrointest Oncol. 2021. PMID: 34457187 Free PMC article. Review.
-
Stepping forward in antibody-drug conjugate development.Pharmacol Ther. 2022 Jan;229:107917. doi: 10.1016/j.pharmthera.2021.107917. Epub 2021 Jun 24. Pharmacol Ther. 2022. PMID: 34171334 Free PMC article. Review.
-
Trop-2 protein as a therapeutic _target: A focused review on Trop-2-based antibody-drug conjugates and their predictive biomarkers.Bosn J Basic Med Sci. 2022 Feb 1;22(1):14-21. doi: 10.17305/bjbms.2021.6100. Bosn J Basic Med Sci. 2022. PMID: 34181512 Free PMC article. Review.
-
The recent progress of peptide regulators for the Wnt/β-catenin signaling pathway.Front Med (Lausanne). 2023 Jun 16;10:1164656. doi: 10.3389/fmed.2023.1164656. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37396899 Free PMC article. Review.
References
-
- Nejadmoghaddam MR, Babamahmoodi A, Minai-Tehrani A, Zarnani AH, Dinarvand R. The use of objective oriented project planning tools for nanosafety and health concerns: a case study in nanomedicine research project. Eur J Nanomed 2016;8(4):225–231.
-
- Decarvalho S, Rand HJ, Lewis A. Coupling of cyclic chemotherapeutic compounds to immune gamma-globulins. Nature 1964;202(4929):255–258. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
