Persistent motor dysfunction despite homeostatic rescue of cerebellar morphogenesis in the Car8 waddles mutant mouse
- PMID: 30867000
- PMCID: PMC6417138
- DOI: 10.1186/s13064-019-0130-4
Persistent motor dysfunction despite homeostatic rescue of cerebellar morphogenesis in the Car8 waddles mutant mouse
Abstract
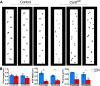
Background: Purkinje cells play a central role in establishing the cerebellar circuit. Accordingly, disrupting Purkinje cell development impairs cerebellar morphogenesis and motor function. In the Car8wdl mouse model of hereditary ataxia, severe motor deficits arise despite the cerebellum overcoming initial defects in size and morphology.
Methods: To resolve how this compensation occurs, we asked how the loss of carbonic anhydrase 8 (CAR8), a regulator of IP3R1 Ca2+ signaling in Purkinje cells, alters cerebellar development in Car8wdl mice. Using a combination of histological, physiological, and behavioral analyses, we determined the extent to which the loss of CAR8 affects cerebellar anatomy, neuronal firing, and motor coordination during development.
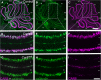
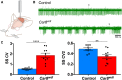
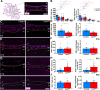
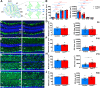
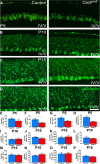
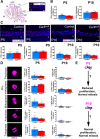
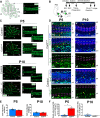
Results: Our results reveal that granule cell proliferation is reduced in early postnatal mutants, although by the third postnatal week there is enhanced and prolonged proliferation, plus an upregulation of Sox2 expression in the inner EGL. Modified circuit patterning of Purkinje cells and Bergmann glia accompany these granule cell adjustments. We also find that although anatomy eventually normalizes, the abnormal activity of neurons and muscles persists.
Conclusions: Our data show that losing CAR8 only transiently restricts cerebellar growth, but permanently damages its function. These data support two current hypotheses about cerebellar development and disease: (1) Sox2 expression may be upregulated at sites of injury and contribute to the rescue of cerebellar structure and (2) transient delays to developmental processes may precede permanent motor dysfunction. Furthermore, we characterize waddles mutant mouse morphology and behavior during development and propose a Sox2-positive, cell-mediated role for rescue in a mouse model of human motor diseases.
Keywords: Ataxia; Dystonia; Granule cell; Lobule; Proliferation; Purkinje cell; Stem cells; Tremor.
Conflict of interest statement
Ethics approval and consent to participate
All animal studies were carried out under an approved Institutional Animal Care and Use Committee (IACUC) animal protocol according to the institutional guidelines at Baylor College of Medicine (BCM).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Climbing Fiber Development Is Impaired in Postnatal Car8 wdl Mice.Cerebellum. 2018 Feb;17(1):56-61. doi: 10.1007/s12311-017-0886-1. Cerebellum. 2018. PMID: 28940157 Free PMC article.
-
Mice deficient in carbonic anhydrase type 8 exhibit motor dysfunctions and abnormal calcium dynamics in the somatic region of cerebellar granule cells.Behav Brain Res. 2015 Jun 1;286:11-6. doi: 10.1016/j.bbr.2015.02.035. Epub 2015 Feb 23. Behav Brain Res. 2015. PMID: 25721739
-
Carbonic anhydrase-related protein VIII deficiency is associated with a distinctive lifelong gait disorder in waddles mice.Genetics. 2005 Nov;171(3):1239-46. doi: 10.1534/genetics.105.044487. Epub 2005 Aug 22. Genetics. 2005. PMID: 16118194 Free PMC article.
-
Calcium Signaling, PKC Gamma, IP3R1 and CAR8 Link Spinocerebellar Ataxias and Purkinje Cell Dendritic Development.Curr Neuropharmacol. 2018 Jan 30;16(2):151-159. doi: 10.2174/1570159X15666170529104000. Curr Neuropharmacol. 2018. PMID: 28554312 Free PMC article. Review.
-
Interactions Between Purkinje Cells and Granule Cells Coordinate the Development of Functional Cerebellar Circuits.Neuroscience. 2021 May 10;462:4-21. doi: 10.1016/j.neuroscience.2020.06.010. Epub 2020 Jun 14. Neuroscience. 2021. PMID: 32554107 Free PMC article. Review.
Cited by
-
Neuromodulation of the cerebellum rescues movement in a mouse model of ataxia.Nat Commun. 2021 Feb 26;12(1):1295. doi: 10.1038/s41467-021-21417-8. Nat Commun. 2021. PMID: 33637754 Free PMC article.
-
Glutamatergic cerebellar neurons differentially contribute to the acquisition of motor and social behaviors.Nat Commun. 2023 May 15;14(1):2771. doi: 10.1038/s41467-023-38475-9. Nat Commun. 2023. PMID: 37188723 Free PMC article.
-
Identification of novel genetic loci and candidate genes for progressive ethanol consumption in diversity outbred mice.Neuropsychopharmacology. 2024 Nov;49(12):1892-1904. doi: 10.1038/s41386-024-01902-6. Epub 2024 Jun 29. Neuropsychopharmacology. 2024. PMID: 38951586 Free PMC article.
-
Propranolol Modulates Cerebellar Circuit Activity and Reduces Tremor.Cells. 2022 Dec 1;11(23):3889. doi: 10.3390/cells11233889. Cells. 2022. PMID: 36497147 Free PMC article.
-
Cerebellar dysfunction in rodent models with dystonia, tremor, and ataxia.Dystonia. 2023;2:11515. doi: 10.3389/dyst.2023.11515. Epub 2023 Dec 8. Dystonia. 2023. PMID: 38105800 Free PMC article.
References
-
- Ledoux MS, Lorden JF. Abnormal spontaneous and harmaline-stimulated Purkinje cell activity in the awake genetically dystonic rat. Exp Brain Res. 2002;145:457–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

