Evaluation of Polymer Nanoformulations in Hepatoma Therapy by Established Rodent Models
- PMID: 30867842
- PMCID: PMC6401493
- DOI: 10.7150/thno.31683
Evaluation of Polymer Nanoformulations in Hepatoma Therapy by Established Rodent Models
Abstract
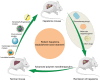
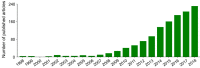
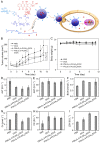
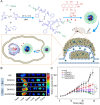
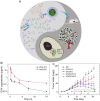
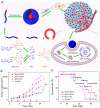
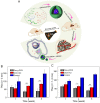
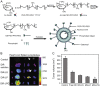
Hepatoma is one of the most severe malignancies usually with poor prognosis, and many patients are insensitive to the existing therapeutic agents, including the drugs for chemotherapy and molecular _targeted therapy. Currently, researchers are committed to developing the advanced formulations with controlled drug delivery to improve the efficacy of hepatoma therapy. Numerous inoculated, induced, and genetically engineered hepatoma rodent models are now available for formulation screening. However, animal models of hepatoma cannot accurately represent human hepatoma in terms of histological characteristics, metastatic pathways, and post-treatment responses. Therefore, advanced animal hepatoma models with comparable pathogenesis and pathological features are in urgent need in the further studies. Moreover, the development of nanomedicines has renewed hope for chemotherapy and molecular _targeted therapy of advanced hepatoma. As one kind of advanced formulations, the polymer-based nanoformulated drugs have many advantages over the traditional ones, such as improved tumor selectivity and treatment efficacy, and reduced systemic side effects. In this article, the construction of rodent hepatoma model and much information about the current development of polymer nanomedicines were reviewed in order to provide a basis for the development of advanced formulations with clinical therapeutic potential for hepatoma.
Keywords: chemotherapy; drug delivery; hepatoma; molecular _targeted therapy; polymer nanoparticle; rodent model.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
Folate-_targeted paclitaxel-conjugated polymeric micelles inhibits pulmonary metastatic hepatoma in experimental murine H22 metastasis models.Int J Nanomedicine. 2014 Apr 23;9:2019-30. doi: 10.2147/IJN.S57744. eCollection 2014. Int J Nanomedicine. 2014. PMID: 24790440 Free PMC article.
-
Polymer nanostructures synthesized by controlled living polymerization for tumor-_targeted drug delivery.J Control Release. 2015 Dec 10;219:345-354. doi: 10.1016/j.jconrel.2015.08.054. Epub 2015 Sep 2. J Control Release. 2015. PMID: 26342661 Free PMC article. Review.
-
Advanced _targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy.Eur J Pharm Biopharm. 2015 Jun;93:52-79. doi: 10.1016/j.ejpb.2015.03.018. Epub 2015 Mar 23. Eur J Pharm Biopharm. 2015. PMID: 25813885 Review.
-
Dual-molecular _targeting nanomedicine upregulates synergistic therapeutic efficacy in preclinical hepatoma models.Acta Biomater. 2024 Jul 15;183:306-317. doi: 10.1016/j.actbio.2024.05.045. Epub 2024 Jun 3. Acta Biomater. 2024. PMID: 38838902
-
Intracellularly Swollen Polypeptide Nanogel Assists Hepatoma Chemotherapy.Theranostics. 2017 Jan 15;7(3):703-716. doi: 10.7150/thno.16794. eCollection 2017. Theranostics. 2017. PMID: 28255361 Free PMC article.
Cited by
-
Nanomaterials modulate tumor-associated macrophages for the treatment of digestive system tumors.Bioact Mater. 2024 Mar 20;36:376-412. doi: 10.1016/j.bioactmat.2024.03.003. eCollection 2024 Jun. Bioact Mater. 2024. PMID: 38544737 Free PMC article. Review.
-
Shear Speed-Regulated Properties of Long-Acting Docetaxel Control Release Poly (Lactic-Co-Glycolic Acid) Microspheres.Front Pharmacol. 2020 Aug 20;11:1286. doi: 10.3389/fphar.2020.01286. eCollection 2020. Front Pharmacol. 2020. PMID: 32973517 Free PMC article.
-
Role and Mechanism of Rhizopus Nigrum Polysaccharide EPS1-1 as Pharmaceutical for Therapy of Hepatocellular Carcinoma.Front Bioeng Biotechnol. 2020 Jun 9;8:509. doi: 10.3389/fbioe.2020.00509. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32582655 Free PMC article.
-
Nanomaterials Enhance the Immunomodulatory Effect of Molecular _targeted Therapy.Int J Nanomedicine. 2021 Mar 1;16:1631-1661. doi: 10.2147/IJN.S290346. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33688183 Free PMC article. Review.
-
Regulatory mechanisms and potential medical applications of HNF1A-AS1 in cancers.Am J Transl Res. 2022 Jun 15;14(6):4154-4168. eCollection 2022. Am J Transl Res. 2022. PMID: 35836869 Free PMC article. Review.
References
-
- Thomas MB, Zhu AX. Hepatocellular carcinoma: The need for progress. J Clin Oncol. 2005;23:2892–9. - PubMed
-
- Vogel A, Cervantes A, Chau I, Daniele B, Llovet J, Meyer T. et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:238–55. - PubMed
-
- Chen J, Ding J, Xiao C, Zhuang X, Chen X. Emerging antitumor applications of extracellularly reengineered polymeric nanocarriers. Biomater Sci. 2015;3:988–1001. - PubMed
-
- Ding J, Shi F, Li D, Chen L, Zhuang X, Chen X. Enhanced endocytosis of acid-sensitive doxorubicin derivatives with intelligent nanogel for improved security and efficacy. Biomater Sci. 2013;1:633–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

