Endothelial cells are a source of Nestin expression in Pulmonary Arterial Hypertension
- PMID: 30883593
- PMCID: PMC6422269
- DOI: 10.1371/journal.pone.0213890
Endothelial cells are a source of Nestin expression in Pulmonary Arterial Hypertension
Abstract
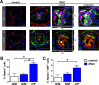
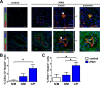
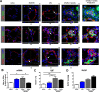
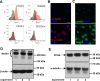
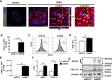
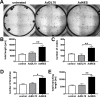
Uncontrolled proliferation of endothelial cells is essential to the pathogenesis of pulmonary arterial hypertension (PAH). Both proliferation and cytoskeleton reorganization are associated with upregulation of the intermediate filament protein Nestin. Recently, accumulation of Nestin-expressing cells was found in pulmonary vascular lesions of PAH patients. The goal of this study is to determine if Nestin expression contributes to endothelial proliferation in pulmonary arterial hypertension, using both lung tissues and endothelial cells. Here we found that endothelial cells from complex and plexiform lesions of PAH patients expressed Nestin. These Nestin+ cells further stained positive for the angiogenic factors CXC chemokine ligand 12 and Wnt1. Likewise, in the chronic hypoxia/SU5416 animal model of pulmonary hypertension, Nestin+ endothelial cells were found in occlusive pulmonary vascular lesions. In vitro, both growing rat and human lung endothelial cells expressed Nestin protein. When Nestin was overexpressed in endothelial cells (both rat and human), Nestin overexpression promoted proliferation and expression of CXC chemokine ligand 12. Nestin overexpression further increased angiogenic tube formation in vitro. Conclusions: We found increased Nestin expression from endothelial cells of occlusive lung vascular lesions in severe pulmonary hypertension. Elevated Nestin expression likely contributes to unchecked pulmonary vascular proliferation and angiogenesis, possibly via induction of CXC chemokine ligand 12. Additional studies are required to determine whether _targeting Nestin would be beneficial to treat PAH.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Nestin represents a potential marker of pulmonary vascular remodeling in pulmonary arterial hypertension associated with congenital heart disease.J Mol Cell Cardiol. 2020 Dec;149:41-53. doi: 10.1016/j.yjmcc.2020.09.005. Epub 2020 Sep 18. J Mol Cell Cardiol. 2020. PMID: 32950539
-
Plexiform-like lesions and increased tissue factor expression in a rat model of severe pulmonary arterial hypertension.Am J Physiol Lung Cell Mol Physiol. 2007 Sep;293(3):L583-90. doi: 10.1152/ajplung.00321.2006. Epub 2007 Jun 22. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17586694
-
Neutrophil Extracellular Traps Promote Angiogenesis: Evidence From Vascular Pathology in Pulmonary Hypertension.Arterioscler Thromb Vasc Biol. 2016 Oct;36(10):2078-87. doi: 10.1161/ATVBAHA.116.307634. Epub 2016 Jul 28. Arterioscler Thromb Vasc Biol. 2016. PMID: 27470511
-
Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation.Respir Res. 2009 Oct 13;10(1):95. doi: 10.1186/1465-9921-10-95. Respir Res. 2009. PMID: 19825167 Free PMC article. Review.
-
Endothelial progenitor cells in pulmonary arterial hypertension.Trends Cardiovasc Med. 2010 Jan;20(1):22-9. doi: 10.1016/j.tcm.2010.03.003. Trends Cardiovasc Med. 2010. PMID: 20685574 Review.
Cited by
-
Clonally selected primitive endothelial cells promote occlusive pulmonary arteriopathy and severe pulmonary hypertension in rats exposed to chronic hypoxia.Sci Rep. 2020 Jan 24;10(1):1136. doi: 10.1038/s41598-020-58083-7. Sci Rep. 2020. PMID: 31980720 Free PMC article.
-
Nestin-Expressing Cells in the Lung: The Bad and the Good Parts.Cells. 2021 Dec 4;10(12):3413. doi: 10.3390/cells10123413. Cells. 2021. PMID: 34943921 Free PMC article. Review.
-
Recent Developments in Nanomaterials-Based Drug Delivery and Upgrading Treatment of Cardiovascular Diseases.Int J Mol Sci. 2022 Jan 26;23(3):1404. doi: 10.3390/ijms23031404. Int J Mol Sci. 2022. PMID: 35163328 Free PMC article. Review.
-
Dichotomous role of integrin-β5 in lung endothelial cells.Pulm Circ. 2022 Oct 1;12(4):e12156. doi: 10.1002/pul2.12156. eCollection 2022 Oct. Pulm Circ. 2022. PMID: 36438452 Free PMC article.
-
Using extracellular matrix derived from sugen-chronic hypoxia lung tissue to study pulmonary arterial hypertension.Front Pharmacol. 2023 Sep 5;14:1192798. doi: 10.3389/fphar.2023.1192798. eCollection 2023. Front Pharmacol. 2023. PMID: 37731734 Free PMC article.
References
-
- Yeager ME, Halley GR, Golpon HA, Voelkel NF, Tuder RM (2001) Microsatellite instability of endothelial cell growth and apoptosis genes within plexiform lesions in primary pulmonary hypertension. Circ Res 88: E2–e11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

