Gene-expression profiling of bortezomib added to standard chemoimmunotherapy for diffuse large B-cell lymphoma (REMoDL-B): an open-label, randomised, phase 3 trial
- PMID: 30948276
- PMCID: PMC6494978
- DOI: 10.1016/S1470-2045(18)30935-5
Gene-expression profiling of bortezomib added to standard chemoimmunotherapy for diffuse large B-cell lymphoma (REMoDL-B): an open-label, randomised, phase 3 trial
Abstract
Background: Biologically distinct subtypes of diffuse large B-cell lymphoma can be identified using gene-expression analysis to determine their cell of origin, corresponding to germinal centre or activated B cell. We aimed to investigate whether adding bortezomib to standard therapy could improve outcomes in patients with these subtypes.
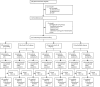
Methods: In a randomised evaluation of molecular guided therapy for diffuse large B-cell lymphoma with bortezomib (REMoDL-B), an open-label, adaptive, randomised controlled, phase 3 superiority trial, participants were recruited from 107 cancer centres in the UK (n=94) and Switzerland (n=13). Eligible patients had previously untreated, histologically confirmed diffuse large B-cell lymphoma with sufficient diagnostic material from initial biopsies for gene-expression profiling and pathology review; were aged 18 years or older; had ECOG performance status of 2 or less; had bulky stage I or stage II-IV disease requiring full-course chemotherapy; had measurable disease; and had cardiac, lung, renal, and liver function sufficient to tolerate chemotherapy. Patients initially received one 21-day cycle of standard rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP; rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, and vincristine 1·4 mg/m2 [to a maximum of 2 mg total dose] intravenously on day 1 of the cycle, and prednisolone 100 mg orally once daily on days 1-5). During this time, we did gene-expression profiling using whole genome cDNA-mediated annealing, selection, extension, and ligation assay of tissue from routine diagnostic biopsy samples to determine the cell-of-origin subtype of each participant (germinal centre B cell, activated B cell, or unclassified). Patients were then centrally randomly assigned (1:1) via a web-based system, with block randomisation stratified by international prognostic index score and cell-of-origin subtype, to continue R-CHOP alone (R-CHOP group; control), or with bortezomib (RB-CHOP group; experimental; 1·3 mg/m2 intravenously or 1·6 mg/m2 subcutaneously) on days 1 and 8 for cycles two to six. If RNA extracted from the diagnostic tissues was of insufficient quality or quantity, participants were given R-CHOP as per the control group. The primary endpoint was 30-month progression-free survival, for the germinal centre and activated B-cell population. The primary analysis was on the modified intention-to-treat population of activated and germinal centre B-cell population. Safety was assessed in all participants who were given at least one dose of study drug. We report the progression-free survival and safety outcomes for patients in the follow-up phase after the required number of events occurred. This study was registered at ClinicalTrials.gov, number NCT01324596, and recruitment and treatment has completed for all participants, with long-term follow-up ongoing.
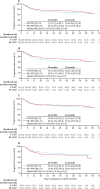
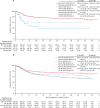
Findings: Between June 2, 2011, and June 10, 2015, 1128 eligible patients were registered, of whom 918 (81%) were randomly assigned to receive treatment (n=459 to R-CHOP, n=459 to RB-CHOP), comprising 244 (26·6%) with activated B-cell disease, 475 (51·7%) with germinal centre B cell disease, and 199 (21·7%) with unclassified disease. At a median follow-up of 29·7 months (95% CI 29·0-32·0), we saw no evidence for a difference in progression-free survival in the combined germinal centre and activated B-cell population between R-CHOP and RB-CHOP (30-month progression-free survival 70·1%, 95% CI 65·0-74·7 vs 74·3%, 69·3-78·7; hazard ratio 0·86, 95% CI 0·65-1·13; p=0·28). The most common grade 3 or worse adverse event was haematological toxicity, reported in 178 (39·8%) of 447 patients given R-CHOP and 187 (42·1%) of 444 given RB-CHOP. However, RB-CHOP was not associated with increased haematological toxicity and 398 [87·1%] of 459 participants assigned to receive RB-CHOP completed six cycles of treatment. Grade 3 or worse neuropathy occurred in 17 (3·8%) patients given RB-CHOP versus eight (1·8%) given R-CHOP. Serious adverse events occurred in 190 (42·5%) patients given R-CHOP, including five treatment-related deaths, and 223 (50·2%) given RB-CHOP, including four treatment-related deaths.
Interpretation: This is the first large-scale study in diffuse large B-cell lymphoma to use real-time molecular characterisation for prospective stratification, randomisation, and subsequent analysis of biologically distinct subgroups of patients. The addition of bortezomib did not improve progression-free survival.
Funding: Janssen-Cilag, Bloodwise, and Cancer Research UK.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Improving R-CHOP in diffuse large B-cell lymphoma is still a challenge.Lancet Oncol. 2019 May;20(5):605-606. doi: 10.1016/S1470-2045(19)30021-X. Epub 2019 Apr 1. Lancet Oncol. 2019. PMID: 30948275 No abstract available.
Similar articles
-
Frontline bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in transplantation-ineligible patients with newly diagnosed mantle cell lymphoma: final overall survival results of a randomised, open-label, phase 3 study.Lancet Oncol. 2018 Nov;19(11):1449-1458. doi: 10.1016/S1470-2045(18)30685-5. Epub 2018 Oct 19. Lancet Oncol. 2018. PMID: 30348538 Clinical Trial.
-
Rituximab-dose-dense chemotherapy with or without high-dose chemotherapy plus autologous stem-cell transplantation in high-risk diffuse large B-cell lymphoma (DLCL04): final results of a multicentre, open-label, randomised, controlled, phase 3 study.Lancet Oncol. 2017 Aug;18(8):1076-1088. doi: 10.1016/S1470-2045(17)30444-8. Epub 2017 Jun 28. Lancet Oncol. 2017. PMID: 28668386 Clinical Trial.
-
Four versus six cycles of CHOP chemotherapy in combination with six applications of rituximab in patients with aggressive B-cell lymphoma with favourable prognosis (FLYER): a randomised, phase 3, non-inferiority trial.Lancet. 2019 Dec 21;394(10216):2271-2281. doi: 10.1016/S0140-6736(19)33008-9. Lancet. 2019. PMID: 31868632 Clinical Trial.
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
-
Limited-stage diffuse large B-cell lymphoma: current management and challenges.Br J Haematol. 2021 Aug;194(3):508-517. doi: 10.1111/bjh.17359. Epub 2021 Feb 22. Br J Haematol. 2021. PMID: 33618434 Review.
Cited by
-
Case report: Successful management of a refractory double-expressor diffuse large B-cell lymphoma patient under the guidance of in vitro high-throughput drug sensitivity test.Front Oncol. 2023 Jan 18;12:1079890. doi: 10.3389/fonc.2022.1079890. eCollection 2022. Front Oncol. 2023. PMID: 36741708 Free PMC article.
-
Biological and Clinical Implications of Gene-Expression Profiling in Diffuse Large B-Cell Lymphoma: A Proposal for a _targeted BLYM-777 Consortium Panel as Part of a Multilayered Analytical Approach.Cancers (Basel). 2022 Apr 7;14(8):1857. doi: 10.3390/cancers14081857. Cancers (Basel). 2022. PMID: 35454765 Free PMC article. Review.
-
_targeting MYC-driven lymphoma: lessons learned and future directions.Cancer Drug Resist. 2023 Apr 12;6(2):205-222. doi: 10.20517/cdr.2022.127. eCollection 2023. Cancer Drug Resist. 2023. PMID: 37457123 Free PMC article. Review.
-
Pseudotemporal ordering of spatial lymphoid tissue microenvironment profiles trails Unclassified DLBCL at the periphery of the follicle.Front Immunol. 2023 Aug 23;14:1207959. doi: 10.3389/fimmu.2023.1207959. eCollection 2023. Front Immunol. 2023. PMID: 37680642 Free PMC article.
-
Phase 1 study of oral azacitidine (CC-486) plus R-CHOP in previously untreated intermediate- to high-risk DLBCL.Blood. 2022 Feb 24;139(8):1147-1159. doi: 10.1182/blood.2021011679. Blood. 2022. PMID: 34428285 Free PMC article. Clinical Trial.
References
-
- Coiffier B, Lepage E, Briere J. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. - PubMed
-
- Cunningham D, Hawkes EA, Jack A. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381:1817–1826. - PubMed
-
- van Imhoff GW, McMillan A, Matasar MJ. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-cell lymphoma: the ORCHARRD study. J Clin Oncol. 2016;35:544–551. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

