Chitin Deacetylases: Structures, Specificities, and Biotech Applications
- PMID: 30966387
- PMCID: PMC6415152
- DOI: 10.3390/polym10040352
Chitin Deacetylases: Structures, Specificities, and Biotech Applications
Abstract
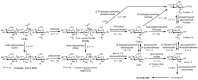
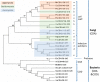
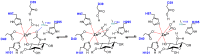
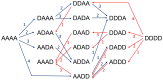
Depolymerization and de-N-acetylation of chitin by chitinases and deacetylases generates a series of derivatives including chitosans and chitooligosaccharides (COS), which are involved in molecular recognition events such as modulation of cell signaling and morphogenesis, immune responses, and host-pathogen interactions. Chitosans and COS are also attractive scaffolds for the development of bionanomaterials for drug/gene delivery and tissue engineering applications. Most of the biological activities associated with COS seem to be largely dependent not only on the degree of polymerization but also on the acetylation pattern, which defines the charge density and distribution of GlcNAc and GlcNH₂ moieties in chitosans and COS. Chitin de-N-acetylases (CDAs) catalyze the hydrolysis of the acetamido group in GlcNAc residues of chitin, chitosan, and COS. The deacetylation patterns are diverse, some CDAs being specific for single positions, others showing multiple attack, processivity or random actions. This review summarizes the current knowledge on substrate specificity of bacterial and fungal CDAs, focusing on the structural and molecular aspects of their modes of action. Understanding the structural determinants of specificity will not only contribute to unravelling structure-function relationships, but also to use and engineer CDAs as biocatalysts for the production of tailor-made chitosans and COS for a growing number of applications.
Keywords: carbohydrate esterases; chitin deacetylases; chitooligosaccharides; chitosan; deacetylation pattern; structure; substrate specificity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Structural basis of chitin oligosaccharide deacetylation.Angew Chem Int Ed Engl. 2014 Jul 1;53(27):6882-7. doi: 10.1002/anie.201400220. Epub 2014 May 8. Angew Chem Int Ed Engl. 2014. PMID: 24810719
-
A Recombinant Fungal Chitin Deacetylase Produces Fully Defined Chitosan Oligomers with Novel Patterns of Acetylation.Appl Environ Microbiol. 2016 Oct 27;82(22):6645-6655. doi: 10.1128/AEM.01961-16. Print 2016 Nov 15. Appl Environ Microbiol. 2016. PMID: 27590819 Free PMC article.
-
Substrate Recognition and Specificity of Chitin Deacetylases and Related Family 4 Carbohydrate Esterases.Int J Mol Sci. 2018 Jan 30;19(2):412. doi: 10.3390/ijms19020412. Int J Mol Sci. 2018. PMID: 29385775 Free PMC article. Review.
-
Screening Assay for Directed Evolution of Chitin Deacetylases: Application to Vibrio cholerae Deacetylase Mutant Libraries for Engineered Specificity.Anal Chem. 2018 Sep 18;90(18):10654-10658. doi: 10.1021/acs.analchem.8b02729. Epub 2018 Aug 28. Anal Chem. 2018. PMID: 30134658
-
The cell factory approach toward biotechnological production of high-value chitosan oligomers and their derivatives: an update.Crit Rev Biotechnol. 2017 Feb;37(1):11-25. doi: 10.3109/07388551.2015.1104289. Epub 2015 Nov 2. Crit Rev Biotechnol. 2017. PMID: 26526199 Review.
Cited by
-
Discovery of Chitin Deacetylase Inhibitors through Structure-Based Virtual Screening and Biological Assays.J Microbiol Biotechnol. 2022 Apr 28;32(4):504-513. doi: 10.4014/jmb.2201.01009. J Microbiol Biotechnol. 2022. PMID: 35131956 Free PMC article.
-
Eco-friendly synthesis of chitosan and its medical application: from chitin extraction to nanoparticle preparation.ADMET DMPK. 2023 Sep 23;11(4):435-455. doi: 10.5599/admet.1999. eCollection 2023. ADMET DMPK. 2023. PMID: 37937250 Free PMC article. Review.
-
Chitin and chitosan remodeling defines vegetative development and Trichoderma biocontrol.PLoS Pathog. 2020 Feb 20;16(2):e1008320. doi: 10.1371/journal.ppat.1008320. eCollection 2020 Feb. PLoS Pathog. 2020. PMID: 32078661 Free PMC article.
-
Enhanced Chitin Deacetylase Production Ability of Rhodococcus equi CGMCC14861 by Co-culture Fermentation With Staphylococcus sp. MC7.Front Microbiol. 2020 Dec 10;11:592477. doi: 10.3389/fmicb.2020.592477. eCollection 2020. Front Microbiol. 2020. PMID: 33362742 Free PMC article.
-
Exopolysaccharides from bacteria and fungi: current status and perspectives in Africa.Heliyon. 2020 Jun 15;6(6):e04205. doi: 10.1016/j.heliyon.2020.e04205. eCollection 2020 Jun. Heliyon. 2020. PMID: 32577572 Free PMC article. Review.
References
-
- Peniche Covas C.A., Argüelles-Monal W., Goycoolea F.M. Chitin and chitosan: Major sources, properties and applications. In: Belgacem M.N., Gandini A., editors. Monomers, Polymers and Composites from Renewable Resources. Volume 1. Elsevier; Amsterdam, The Netherlands: 2008. pp. 517–542.
-
- Karrer P., Hofmann A. Über den enzymatischen Abbau von Chitin und Chitosan I. Helv. Chim. Acta. 1929;12:616–637. doi: 10.1002/hlca.19290120167. - DOI
-
- Rinaudo M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006;31:603–632. doi: 10.1016/j.progpolymsci.2006.06.001. - DOI
-
- Dutta P.K., Duta J., Tripathi V.S. Chitin and Chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. (India) 2004;63:20–31. doi: 10.1002/chin.200727270. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

