Novel Contributors and Mechanisms of Cellular Senescence in Hypertension-Associated Premature Vascular Aging
- PMID: 30982879
- PMCID: PMC6636693
- DOI: 10.1093/ajh/hpz052
Novel Contributors and Mechanisms of Cellular Senescence in Hypertension-Associated Premature Vascular Aging
Abstract
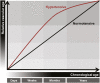
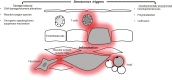
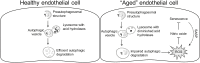
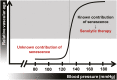
Hypertension has been described as a condition of premature vascular aging, relative to actual chronological age. In fact, many factors that contribute to the deterioration of vascular function as we age are accelerated in hypertension. Nonetheless, the precise mechanisms that underlie the aged phenotype of arteries from hypertensive patients and animals remain elusive. Cellular senescence is an age-related physiologic process in which cells undergo irreversible growth arrest. Although controlled senescence negatively regulates cell proliferation and promotes tissue regeneration, uncontrolled senescence can contribute to disease pathogenesis by presenting the senescence-associated secretory phenotype, in which molecules such as proinflammatory cytokines, matrix metalloproteases, and reactive oxygen species are released into tissue microenvironments. This review will address and critically evaluate the current literature on the role of cellular senescence in hypertension, with particular emphasis on cells types that mediate and modulate vascular function and structure.
Keywords: blood pressure; cellular senescence; hypertension; vascular aging.
© American Journal of Hypertension, Ltd 2019. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Mitophagy in Hypertension-Associated Premature Vascular Aging.Am J Hypertens. 2020 Sep 10;33(9):804-812. doi: 10.1093/ajh/hpaa058. Am J Hypertens. 2020. PMID: 32533696 Free PMC article. Review.
-
Vascular smooth muscle cell senescence and age-related diseases: State of the art.Biochim Biophys Acta Mol Basis Dis. 2019 Jul 1;1865(7):1810-1821. doi: 10.1016/j.bbadis.2018.08.015. Epub 2018 Aug 16. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 31109451 Review.
-
Induced Trf2 deletion leads to aging vascular phenotype in mice associated with arterial telomere uncapping, senescence signaling, and oxidative stress.J Mol Cell Cardiol. 2019 Feb;127:74-82. doi: 10.1016/j.yjmcc.2018.11.014. Epub 2018 Nov 29. J Mol Cell Cardiol. 2019. PMID: 30502348 Free PMC article.
-
Mechanisms of Vascular Aging.Circ Res. 2018 Sep 14;123(7):849-867. doi: 10.1161/CIRCRESAHA.118.311378. Circ Res. 2018. PMID: 30355080 Free PMC article. Review.
-
Microvascular Alterations in Hypertension and Vascular Aging.Curr Hypertens Rev. 2017;13(1):16-23. doi: 10.2174/1573402113666170505115010. Curr Hypertens Rev. 2017. PMID: 28474545
Cited by
-
_targeting Molecular Mechanism of Vascular Smooth Muscle Senescence Induced by Angiotensin II, A Potential Therapy via Senolytics and Senomorphics.Int J Mol Sci. 2020 Sep 9;21(18):6579. doi: 10.3390/ijms21186579. Int J Mol Sci. 2020. PMID: 32916794 Free PMC article. Review.
-
Cells in Atherosclerosis: Focus on Cellular Senescence from Basic Science to Clinical Practice.Int J Mol Sci. 2023 Dec 5;24(24):17129. doi: 10.3390/ijms242417129. Int J Mol Sci. 2023. PMID: 38138958 Free PMC article. Review.
-
Immunosenescence in atherosclerosis: A role for chronic viral infections.Front Immunol. 2022 Aug 17;13:945016. doi: 10.3389/fimmu.2022.945016. eCollection 2022. Front Immunol. 2022. PMID: 36059478 Free PMC article. Review.
-
Mitophagy in Hypertension-Associated Premature Vascular Aging.Am J Hypertens. 2020 Sep 10;33(9):804-812. doi: 10.1093/ajh/hpaa058. Am J Hypertens. 2020. PMID: 32533696 Free PMC article. Review.
-
Cellular senescence of renal tubular epithelial cells in acute kidney injury.Cell Death Discov. 2024 Feb 5;10(1):62. doi: 10.1038/s41420-024-01831-9. Cell Death Discov. 2024. PMID: 38316761 Free PMC article. Review.
References
-
- Thomas H. Senescence, ageing and death of the whole plant. New Phytol 2013; 197:696–711. - PubMed
-
- Faconti L, Bruno RM, Ghiadoni L, Taddei S, Virdis A. Ventricular and vascular stiffening in aging and hypertension. Curr Hypertens Rev 2015; 11:100–109. - PubMed
-
- Writing Group Members , Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee, Stroke Statistics Subcommittee . Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016;133:e38–360. - PubMed
-
- Abeywardena MY, Jablonskis LT, Head RJ. Age- and hypertension-induced changes in abnormal contractions in rat aorta. J Cardiovasc Pharmacol 2002; 40:930–937. - PubMed
-
- Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017; 70:660–667. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

