Yap/Taz regulate alveolar regeneration and resolution of lung inflammation
- PMID: 30985294
- PMCID: PMC6486331
- DOI: 10.1172/JCI125014
Yap/Taz regulate alveolar regeneration and resolution of lung inflammation
Abstract
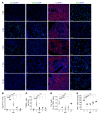
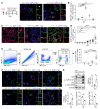
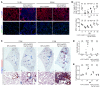
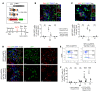
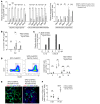
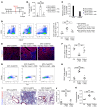
Alveolar epithelium plays a pivotal role in protecting the lungs from inhaled infectious agents. Therefore, the regenerative capacity of the alveolar epithelium is critical for recovery from these insults in order to rebuild the epithelial barrier and restore pulmonary functions. Here, we show that sublethal infection of mice with Streptococcus pneumoniae, the most common pathogen of community-acquired pneumonia, led to exclusive damage in lung alveoli, followed by alveolar epithelial regeneration and resolution of lung inflammation. We show that surfactant protein C-expressing (SPC-expressing) alveolar epithelial type II cells (AECIIs) underwent proliferation and differentiation after infection, which contributed to the newly formed alveolar epithelium. This increase in AECII activities was correlated with increased nuclear expression of Yap and Taz, the mediators of the Hippo pathway. Mice that lacked Yap/Taz in AECIIs exhibited prolonged inflammatory responses in the lung and were delayed in alveolar epithelial regeneration during bacterial pneumonia. This impaired alveolar epithelial regeneration was paralleled by a failure to upregulate IκBa, the molecule that terminates NF-κB-mediated inflammatory responses. These results demonstrate that signals governing resolution of lung inflammation were altered in Yap/Taz mutant mice, which prevented the development of a proper regenerative niche, delaying repair and regeneration of alveolar epithelium during bacterial pneumonia.
Keywords: Adult stem cells; Bacterial infections; Pulmonology; Stem cells.
Conflict of interest statement
Figures

Similar articles
-
Hippo signaling impairs alveolar epithelial regeneration in pulmonary fibrosis.Elife. 2023 May 11;12:e85092. doi: 10.7554/eLife.85092. Elife. 2023. PMID: 37166104 Free PMC article.
-
Epithelial Yap/Taz are required for functional alveolar regeneration following acute lung injury.JCI Insight. 2023 Sep 7;8(19):e173374. doi: 10.1172/jci.insight.173374. JCI Insight. 2023. PMID: 37676731 Free PMC article.
-
Angiocrine Sphingosine-1-Phosphate Activation of S1PR2-YAP Signaling Axis in Alveolar Type II Cells Is Essential for Lung Repair.Cell Rep. 2020 Jun 30;31(13):107828. doi: 10.1016/j.celrep.2020.107828. Cell Rep. 2020. PMID: 32610129 Free PMC article.
-
Mechanisms of ATII-to-ATI Cell Differentiation during Lung Regeneration.Int J Mol Sci. 2020 Apr 30;21(9):3188. doi: 10.3390/ijms21093188. Int J Mol Sci. 2020. PMID: 32366033 Free PMC article. Review.
-
The Hippo-YAP/TAZ Signaling Pathway in Intestinal Self-Renewal and Regeneration After Injury.Front Cell Dev Biol. 2022 Jul 19;10:894737. doi: 10.3389/fcell.2022.894737. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35927987 Free PMC article. Review.
Cited by
-
Pathogenesis and Therapy of Hermansky-Pudlak Syndrome (HPS)-Associated Pulmonary Fibrosis.Int J Mol Sci. 2024 Oct 19;25(20):11270. doi: 10.3390/ijms252011270. Int J Mol Sci. 2024. PMID: 39457053 Free PMC article. Review.
-
A calpain-6/YAP axis in sarcoma stem cells that drives the outgrowth of tumors and metastases.Cell Death Dis. 2022 Sep 24;13(9):819. doi: 10.1038/s41419-022-05244-3. Cell Death Dis. 2022. PMID: 36153320 Free PMC article.
-
Defining signals that promote human alveolar type I differentiation.Am J Physiol Lung Cell Mol Physiol. 2024 Apr 1;326(4):L409-L418. doi: 10.1152/ajplung.00191.2023. Epub 2024 Feb 13. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 38349124
-
Alveolar epithelial progenitor cells require Nkx2-1 to maintain progenitor-specific epigenomic state during lung homeostasis and regeneration.Nat Commun. 2023 Dec 19;14(1):8452. doi: 10.1038/s41467-023-44184-0. Nat Commun. 2023. PMID: 38114516 Free PMC article.
-
25(OH)-Vitamin D alleviates neonatal infectious pneumonia via regulating TGFβ-mediated nuclear translocation mechanism of YAP/TAZ.Bioengineered. 2021 Dec;12(1):8931-8942. doi: 10.1080/21655979.2021.1990000. Bioengineered. 2021. PMID: 34643152 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

