Design, Synthesis, Molecular Modeling, In Silico ADME Studies and Anti-HIV-1 Assay of New Diazocoumarin Derivatives
- PMID: 31011343
- PMCID: PMC6447871
Design, Synthesis, Molecular Modeling, In Silico ADME Studies and Anti-HIV-1 Assay of New Diazocoumarin Derivatives
Abstract
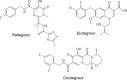
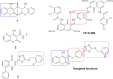
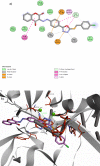
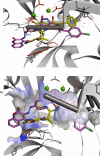
Some new diazo incorporated coumarin compounds were designed and synthesized to evaluate their anti-HIV activity. Overall, compounds were active against HIV at 100 μM. Additionally, no cytotoxic effect was observed at this concentration. The compound with 4-chlorobenzyl group indicated the best anti-HIV activity (52%). Docking studies using the later crystallographic data available for PFV integrase showed similar binding modes to HIV-1 integrase inhibitors. On the basis of these data, nitrogen atoms of 1,3,4-oxadiazole ring have been involved in the Mg2+ chelation and 4-chlorobenzyl group occupies the same position as 4-flourobenzyl group of raltegravir in the active site. In addition, in silico ADME assay demonstrated favorable physicochemical properties for the new designed compounds. Thus, synthesized structures could be introduced as a novel template for designing safe anti-HIV compounds with integrase inhibitory potential.
Keywords: 1; 3; 4-Oxadiazole; ADME; Anti-HIV; Diazocoumarin; Docking; Synthesis.
Figures

Similar articles
-
Molecular Docking and QSAR Study of 2-Benzoxazolinone, Quinazoline and Diazocoumarin Derivatives as Anti-HIV-1 Agents.Iran J Pharm Res. 2019 Summer;18(3):1253-1263. doi: 10.22037/ijpr.2019.1100746. Iran J Pharm Res. 2019. PMID: 32641936 Free PMC article.
-
Design, synthesis and docking studies of new 4-hydroxyquinoline-3-carbohydrazide derivatives as anti-HIV-1 agents.Drug Res (Stuttg). 2013 Apr;63(4):192-7. doi: 10.1055/s-0033-1334964. Epub 2013 Mar 13. Drug Res (Stuttg). 2013. PMID: 23487403
-
Design, Synthesis, Docking Studies and Biological Activities Novel 2,3- Diaryl-4-Quinazolinone Derivatives as Anti-HIV-1 Agents.Curr HIV Res. 2019;17(3):214-222. doi: 10.2174/1570162X17666190911125359. Curr HIV Res. 2019. PMID: 31518225
-
N-hydroxy-substituted 2-aryl acetamide analogs: A novel class of HIV-1 integrase inhibitors.Chem Biol Drug Des. 2017 Oct;90(4):527-534. doi: 10.1111/cbdd.12974. Epub 2017 Apr 26. Chem Biol Drug Des. 2017. PMID: 28294572
-
2-hydroxyisoquinoline-1,3(2H,4H)-diones as inhibitors of HIV-1 integrase and reverse transcriptase RNase H domain: influence of the alkylation of position 4.Eur J Med Chem. 2011 Feb;46(2):535-46. doi: 10.1016/j.ejmech.2010.11.033. Epub 2010 Nov 27. Eur J Med Chem. 2011. PMID: 21185110
Cited by
-
Molecular Docking and QSAR Study of 2-Benzoxazolinone, Quinazoline and Diazocoumarin Derivatives as Anti-HIV-1 Agents.Iran J Pharm Res. 2019 Summer;18(3):1253-1263. doi: 10.22037/ijpr.2019.1100746. Iran J Pharm Res. 2019. PMID: 32641936 Free PMC article.
-
Development and Evaluation of Some Molecular Hybrids of N-(1-Benzylpiperidin-4-yl)-2-((5-phenyl-1,3,4-oxadiazol-2-yl)thio) as Multifunctional Agents to Combat Alzheimer's Disease.ACS Omega. 2023 Mar 2;8(10):9394-9414. doi: 10.1021/acsomega.2c08061. eCollection 2023 Mar 14. ACS Omega. 2023. PMID: 36936338 Free PMC article.
-
Coumarin: An emerging antiviral agent.Heliyon. 2020 Jan 27;6(1):e03217. doi: 10.1016/j.heliyon.2020.e03217. eCollection 2020 Jan. Heliyon. 2020. PMID: 32042967 Free PMC article.
-
Novel 2-(Diphenylmethylidene) Malonic Acid Derivatives as Anti-HIV Agents: Molecular Modeling, Synthesis and Biological Evaluation.Iran J Pharm Res. 2021 Dec 14;21(1):e123827. doi: 10.5812/ijpr.123827. eCollection 2022 Dec. Iran J Pharm Res. 2021. PMID: 35765501 Free PMC article.
-
Design, Synthesis, Molecular Modeling Study and Biological Evaluation of New N'-Arylidene-pyrido [2,3-d]pyrimidine-5-carbohydrazide Derivatives as Anti-HIV-1 Agents.Iran J Pharm Res. 2019 Fall;18(Suppl1):237-248. doi: 10.22037/ijpr.2019.112198.13597. Iran J Pharm Res. 2019. PMID: 32802103 Free PMC article.
References
-
- Kinch MS, Patridge E. An analysis of FDA-approved drugs for infectious disease: HIV/AIDS drugs. Drug Discov. Today . 2014;19:1510–3. - PubMed
-
- Frentz D, Boucher CAB, Van De Vijver DAMC. Temporal changes in the epidemiology of transmission of drug-resistant HIV-1 across the world. AIDS Rev. 2012;14:17–27. - PubMed
LinkOut - more resources
Full Text Sources
