Pro-Nifuroxazide Self-Assembly Leads to Triggerable Nanomedicine for Anti-cancer Therapy
- PMID: 31013055
- PMCID: PMC7066988
- DOI: 10.1021/acsami.9b01343
Pro-Nifuroxazide Self-Assembly Leads to Triggerable Nanomedicine for Anti-cancer Therapy
Abstract
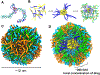
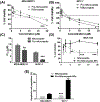
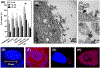
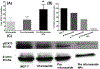
Transcription factor STAT3 has been shown to regulate genes that are involved in stem cell self-renewal and thus represents a novel therapeutic _target of great biological significance. However, many small-molecule agents with potential effects through STAT3 modulation in cancer therapy lack aqueous solubility and high off-_target toxicity, hence impeding efficient bioavailability and activity. This work, for the first time, reports a prodrug-based strategy for selective and safer delivery of STAT3 inhibitors designed toward metastatic and drug-resistant breast cancer. We have synthesized a novel lipase-labile SN-2 phospholipid prodrug from a clinically investigated STAT3 inhibitor, nifuroxazide (Pro-nifuroxazide), which can be regioselectively cleaved by the membrane-abundant enzymes in cancer cells. Pro-nifuroxazide self-assembled to sub 20 nm nanoparticles (NPs), and the cytotoxic ability was screened in ER(+)-MCF-7 and ER(-)-MD-MB231 cells at 48-72 h using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide proliferation assay. Results indicated that Pro-nifuroxazide NPs are multifold more effective toward inhibiting cancer cells in a time-dependent manner compared to parent nifuroxazide. A remarkable improvement in the local concentration of drugs to as high as ∼240 fold when assembled into NPs is presumably the reason for this functional improvement. We also introduced molecular dynamics simulations to generate Pro-nifuroxazide nano-assembly, as a model assembly from triggerable anti-cancer drugs, to provide molecular insights correlating physicochemical and anti-cancer properties. In silico properties of Pro-nifuroxazide including size, chemistry of NPs and membrane interactions with individual molecules could be validated by in vitro functional activities in cells of breast cancer origin. The in vivo anti-cancer efficiencies of Pro-nifuroxazide NPs in nude mice xenografts with MCF-7 revealed remarkable growth inhibition of as high as 400%. Histopathological analysis corroborated these findings to show significantly high nuclear fragmentation and retracted cytoplasm. Immunostaining on tumor section demonstrated a significantly lower level of pSTAT-3 by Pro-nifuroxazide NP treatment, establishing the inhibition of STAT-3 phosphorylation. Our strategy for the first time proposes a translatable prodrug agent self-assembled into NPs and demonstrates remarkable enhancement in IC50, induced apoptosis, and reduced cancer cell population through STAT-3 inhibition via reduced phosphorylation.
Keywords: cancer therapy; dissipative particle dynamics; nanoparticle; prodrug; self-assembly.
Figures



Similar articles
-
ALDH1 Bio-activates Nifuroxazide to Eradicate ALDHHigh Melanoma-Initiating Cells.Cell Chem Biol. 2018 Dec 20;25(12):1456-1469.e6. doi: 10.1016/j.chembiol.2018.09.005. Epub 2018 Oct 4. Cell Chem Biol. 2018. PMID: 30293938 Free PMC article.
-
Inhibition of Stat3 signaling pathway by nifuroxazide improves antitumor immunity and impairs colorectal carcinoma metastasis.Cell Death Dis. 2017 Jan 5;8(1):e2534. doi: 10.1038/cddis.2016.452. Cell Death Dis. 2017. PMID: 28055016 Free PMC article.
-
Nifuroxazide inhibits survival of multiple myeloma cells by directly inhibiting STAT3.Blood. 2008 Dec 15;112(13):5095-102. doi: 10.1182/blood-2007-12-129718. Epub 2008 Sep 29. Blood. 2008. PMID: 18824601 Free PMC article.
-
Toward a repositioning of the antibacterial drug nifuroxazide for cancer treatment.Drug Discov Today. 2019 Sep;24(9):1930-1936. doi: 10.1016/j.drudis.2019.06.017. Epub 2019 Jun 28. Drug Discov Today. 2019. PMID: 31260646 Review.
-
Mipsagargin: The Beginning-Not the End-of Thapsigargin Prodrug-Based Cancer Therapeutics.Molecules. 2021 Dec 9;26(24):7469. doi: 10.3390/molecules26247469. Molecules. 2021. PMID: 34946547 Free PMC article. Review.
Cited by
-
Nifuroxazide Mitigates Angiogenesis in Ehlrich's Solid Carcinoma: Molecular Docking, Bioinformatic and Experimental Studies on Inhibition of Il-6/Jak2/Stat3 Signaling.Molecules. 2021 Nov 13;26(22):6858. doi: 10.3390/molecules26226858. Molecules. 2021. PMID: 34833950 Free PMC article.
-
Application of molecular dynamics simulation in self-assembled cancer nanomedicine.Biomater Res. 2023 May 4;27(1):39. doi: 10.1186/s40824-023-00386-7. Biomater Res. 2023. PMID: 37143168 Free PMC article. Review.
-
Nanoparticulation of Prodrug into Medicines for Cancer Therapy.Adv Sci (Weinh). 2021 Sep;8(18):e2101454. doi: 10.1002/advs.202101454. Epub 2021 Jul 29. Adv Sci (Weinh). 2021. PMID: 34323373 Free PMC article. Review.
-
Redox modulator iron complexes trigger intrinsic apoptosis pathway in cancer cells.iScience. 2024 May 3;27(6):109899. doi: 10.1016/j.isci.2024.109899. eCollection 2024 Jun 21. iScience. 2024. PMID: 38799569 Free PMC article.
-
Evaluation of antioxidant and cytotoxic properties of phenolic N-acylhydrazones: structure-activity relationship.R Soc Open Sci. 2022 Jun 8;9(6):211853. doi: 10.1098/rsos.211853. eCollection 2022 Jun. R Soc Open Sci. 2022. PMID: 35706666 Free PMC article.
References
-
- Ma D; Hettiarachchi G; Nguyen D; Zhang B; Wittenberg JB; Zavalij PY; Briken V; Isaac L Acyclic Cucurbit[n]uril Molecular Containers Enhance the Solubility and Bioactivity of Poorly Soluble Pharmaceuticals. Nat. Chem 2012, 4, 503–510. - PubMed
-
- Gao Z; Lukyanov AN; Singhal A; Torchilin VP Diacyllipid-Polymer Micelles as Nanocarriers for Poorly Soluble Anticancer Drugs. Nano Lett. 2002, 2, 979–982.
-
- Boztas AO; Karakuzu O; Galante G; Ugur Z; Kocabas F; Altuntas CZ; Yazaydin AO Synergistic Interaction of Paclitaxel and Curcumin with Cyclodextrin Polymer Complexation in Human Cancer Cells. Mol. Pharmaceutics 2013, 10, 2676–2683. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

