Spectrum of Trained Innate Immunity Induced by Low-Virulence Candida Species against Lethal Polymicrobial Intra-abdominal Infection
- PMID: 31085710
- PMCID: PMC6652762
- DOI: 10.1128/IAI.00348-19
Spectrum of Trained Innate Immunity Induced by Low-Virulence Candida Species against Lethal Polymicrobial Intra-abdominal Infection
Abstract
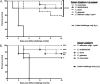
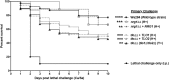
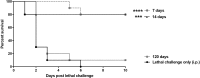
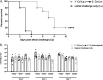
Polymicrobial intra-abdominal infections (IAI) are clinically prevalent and cause significant morbidity and mortality, especially those involving fungi. Our laboratory developed a mouse model of polymicrobial IAI and demonstrated that coinfection with Candida albicans and Staphylococcus aureus (C. albicans/S. aureus) results in 80 to 90% mortality in 48 to 72 h due to robust local and systemic inflammation. Surprisingly, inoculation with Candida dubliniensis and S. aureus resulted in minimal mortality, and rechallenge of mice with lethal C. albicans/S. aureus conferred >90% protection up to 60 days postinoculation. Protection was mediated by Gr-1+ polymorphonuclear leukocytes, indicating a novel form of trained innate immunity (TII). The purpose of this study was to determine the microbial requirements and spectrum of innate-mediated protection. In addition to Candida dubliniensis, several other low-virulence Candida species (C. glabrata, C. auris, and C. albicansefg1Δ/Δ cph1Δ/Δ) and Saccharomyces cerevisiae conferred significant protection with or without S. aureus For C. dubliniensis-mediated protection, hyphal formation was not required, with protection conferred as early as 7 days after primary challenge but not at 120 days, and also following multiple lethal C. albicans/S. aureus rechallenges. This protection also extended to a lethal intravenous (i.v.) C. albicans challenge but had no effect in the C. albicans vaginitis model. Finally, studies revealed the ability of the low-virulence Candida species that conferred protection to invade the bone marrow by 24 h post-primary challenge, with a positive correlation between femoral bone marrow fungal infiltration at 48 h and protection upon rechallenge. These results support and further extend the characterization of this novel TII in protection against lethal fungal-bacterial IAI and sepsis.
Keywords: Candida; intra-abdominal infection; polymicrobial infection; sepsis; trained innate immunity.
Copyright © 2019 Lilly et al.
Figures

Similar articles
-
Metabolic Adaptations During Staphylococcus aureus and Candida albicans Co-Infection.Front Immunol. 2021 Dec 8;12:797550. doi: 10.3389/fimmu.2021.797550. eCollection 2021. Front Immunol. 2021. PMID: 34956233 Free PMC article. Review.
-
Immune Protection against Lethal Fungal-Bacterial Intra-Abdominal Infections.mBio. 2018 Jan 16;9(1):e01472-17. doi: 10.1128/mBio.01472-17. mBio. 2018. PMID: 29339423 Free PMC article.
-
Trained Innate Immunity Induced by Vaccination with Low-Virulence Candida Species Mediates Protection against Several Forms of Fungal Sepsis via Ly6G+ Gr-1+ Leukocytes.mBio. 2021 Oct 26;12(5):e0254821. doi: 10.1128/mBio.02548-21. Epub 2021 Oct 19. mBio. 2021. PMID: 34663098 Free PMC article.
-
Efficacy of Candida dubliniensis and Fungal β-Glucans in Inducing Trained Innate Immune Protection Against Inducers of Sepsis.Front Cell Infect Microbiol. 2022 Jun 13;12:898030. doi: 10.3389/fcimb.2022.898030. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35770067 Free PMC article.
-
Candida/Staphylococcal Polymicrobial Intra-Abdominal Infection: Pathogenesis and Perspectives for a Novel Form of Trained Innate Immunity.J Fungi (Basel). 2019 May 9;5(2):37. doi: 10.3390/jof5020037. J Fungi (Basel). 2019. PMID: 31075836 Free PMC article. Review.
Cited by
-
Could an Unrelated Live Attenuated Vaccine Serve as a Preventive Measure To Dampen Septic Inflammation Associated with COVID-19 Infection?mBio. 2020 Jun 19;11(3):e00907-20. doi: 10.1128/mBio.00907-20. mBio. 2020. PMID: 32561657 Free PMC article.
-
Immunogenicity and efficacy of CNA25 as a potential whole-cell vaccine against systemic candidiasis.EMBO Mol Med. 2024 Jun;16(6):1254-1283. doi: 10.1038/s44321-024-00080-8. Epub 2024 May 23. EMBO Mol Med. 2024. PMID: 38783167 Free PMC article.
-
Reply to Özdemir, "Measles-Mumps-Rubella Vaccine and COVID-19 Relationship".mBio. 2020 Sep 22;11(5):e02465-20. doi: 10.1128/mBio.02465-20. mBio. 2020. PMID: 32963010 Free PMC article. No abstract available.
-
A Fun-Guide to Innate Immune Responses to Fungal Infections.J Fungi (Basel). 2022 Jul 29;8(8):805. doi: 10.3390/jof8080805. J Fungi (Basel). 2022. PMID: 36012793 Free PMC article. Review.
-
Metabolic Adaptations During Staphylococcus aureus and Candida albicans Co-Infection.Front Immunol. 2021 Dec 8;12:797550. doi: 10.3389/fimmu.2021.797550. eCollection 2021. Front Immunol. 2021. PMID: 34956233 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

