Anaerobic Degradation of N-ε-Carboxymethyllysine, a Major Glycation End-Product, by Human Intestinal Bacteria
- PMID: 31091091
- PMCID: PMC6566499
- DOI: 10.1021/acs.jafc.9b02208
Anaerobic Degradation of N-ε-Carboxymethyllysine, a Major Glycation End-Product, by Human Intestinal Bacteria
Abstract
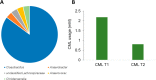
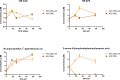
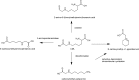
Modifications of lysine contribute to the amount of dietary advanced glycation end-products reaching the colon. However, little is known about the ability of intestinal bacteria to metabolize dietary N-ε-carboxymethyllysine (CML). Successive transfers of fecal microbiota in growth media containing CML were used to identify and isolate species able to metabolize CML under anaerobic conditions. From our study, only donors exposed to processed foods degraded CML, and anaerobic bacteria enrichments from two of them used 77 and 100% of CML. Oscillibacter and Cloacibacillus evryensis increased in the two donors after the second transfer, highlighting that the bacteria from these taxa could be candidates for anaerobic CML degradation. A tentative identification of CML metabolites produced by a pure culture of Cloacibacillus evryensis was performed by mass spectrometry: carboxymethylated biogenic amines and carboxylic acids were identified as CML degradation products. The study confirmed the ability of intestinal bacteria to metabolize CML under anoxic conditions.
Keywords: -ε-carboxymethyllysine; Maillard reaction; dietary advanced glycation end-products; intestinal metabolism; microbiota.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Stability of Individual Maillard Reaction Products in the Presence of the Human Colonic Microbiota.J Agric Food Chem. 2015 Aug 5;63(30):6723-30. doi: 10.1021/acs.jafc.5b01391. Epub 2015 Jul 24. J Agric Food Chem. 2015. PMID: 26186075
-
Metabolization of the Advanced Glycation End Product N-ε-Carboxymethyllysine (CML) by Different Probiotic E. coli Strains.J Agric Food Chem. 2019 Feb 20;67(7):1963-1972. doi: 10.1021/acs.jafc.8b06748. Epub 2019 Feb 12. J Agric Food Chem. 2019. PMID: 30701968
-
Inter- and Intraindividual Differences in the Capacity of the Human Intestinal Microbiome in Fecal Slurries to Metabolize Fructoselysine and Carboxymethyllysine.J Agric Food Chem. 2022 Sep 21;70(37):11759-11768. doi: 10.1021/acs.jafc.2c05756. Epub 2022 Sep 7. J Agric Food Chem. 2022. PMID: 36069406 Free PMC article.
-
Dietary N-epsilon-carboxymethyllysine as for a major glycotoxin in foods: A review.Compr Rev Food Sci Food Saf. 2021 Sep;20(5):4931-4949. doi: 10.1111/1541-4337.12817. Epub 2021 Aug 10. Compr Rev Food Sci Food Saf. 2021. PMID: 34378329 Review.
-
New biomarkers of Maillard reaction damage to proteins.Nephrol Dial Transplant. 1996;11 Suppl 5:41-7. doi: 10.1093/ndt/11.supp5.41. Nephrol Dial Transplant. 1996. PMID: 9044306 Review.
Cited by
-
Ultra-High Temperature Treatment and Storage of Infant Formula Induces Dietary Protein Modifications, Gut Dysfunction, and Inflammation in Preterm Pigs.Mol Nutr Food Res. 2022 Oct;66(20):e2200132. doi: 10.1002/mnfr.202200132. Epub 2022 Sep 12. Mol Nutr Food Res. 2022. PMID: 36052940 Free PMC article.
-
Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs.Cells. 2022 Apr 12;11(8):1312. doi: 10.3390/cells11081312. Cells. 2022. PMID: 35455991 Free PMC article. Review.
-
Dietary Advanced Glycation End-Products and Colorectal Cancer Risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study.Nutrients. 2021 Sep 8;13(9):3132. doi: 10.3390/nu13093132. Nutrients. 2021. PMID: 34579010 Free PMC article.
-
A 4-Week Diet Low or High in Advanced Glycation Endproducts Has Limited Impact on Gut Microbial Composition in Abdominally Obese Individuals: The deAGEing Trial.Int J Mol Sci. 2022 May 10;23(10):5328. doi: 10.3390/ijms23105328. Int J Mol Sci. 2022. PMID: 35628138 Free PMC article. Clinical Trial.
-
Advanced Glycation End Products (AGEs) in Diet and Skin in Relation to Stool Microbiota: The Rotterdam Study.Nutrients. 2023 May 30;15(11):2567. doi: 10.3390/nu15112567. Nutrients. 2023. PMID: 37299529 Free PMC article.
References
-
- Scheijen J. L. J. M.; Clevers E.; Engelen L.; Dagnelie P. C.; Brouns F.; Stehouwer C. D. A.; Schalkwijk C. G. Analysis of Advanced Glycation Endproducts in Selected Food Items by Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry: Presentation of a Dietary AGE Database. Food Chem. 2016, 190, 1145–1150. 10.1016/j.foodchem.2015.06.049. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

