δ-Tocopherol Effect on Endocytosis and Its Combination with Enzyme Replacement Therapy for Lysosomal Disorders: A New Type of Drug Interaction?
- PMID: 31101681
- PMCID: PMC6806345
- DOI: 10.1124/jpet.119.257345
δ-Tocopherol Effect on Endocytosis and Its Combination with Enzyme Replacement Therapy for Lysosomal Disorders: A New Type of Drug Interaction?
Abstract
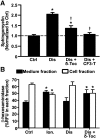
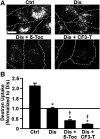
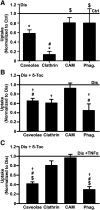
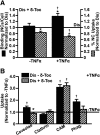
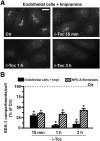
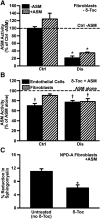
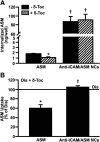
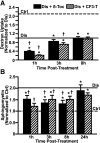
Induction of lysosomal exocytosis alleviates lysosomal storage of undigested metabolites in cell models of lysosomal disorders (LDs). However, whether this strategy affects other vesicular compartments, e.g., those involved in endocytosis, is unknown. This is important both to predict side effects and to use this strategy in combination with therapies that require endocytosis for intracellular delivery, such as lysosomal enzyme replacement therapy (ERT). We investigated this using δ-tocopherol as a model previously shown to induce lysosomal exocytosis and cell models of type A Niemann-Pick disease, a LD characterized by acid sphingomyelinase (ASM) deficiency and sphingomyelin storage. δ-Tocopherol and derivative CF3-T reduced net accumulation of fluid phase, ligands, and polymer particles via phagocytic, caveolae-, clathrin-, and cell adhesion molecule (CAM)-mediated pathways, yet the latter route was less affected due to receptor overexpression. In agreement, δ-tocopherol lowered uptake of recombinant ASM by deficient cells (known to occur via the clathrin pathway) and via _targeting intercellular adhesion molecule-1 (associated to the CAM pathway). However, the net enzyme activity delivered and lysosomal storage attenuation were greater via the latter route. Data suggest stimulation of exocytosis by tocopherols is not specific of lysosomes and affects endocytic cargo. However, this effect was transient and became unnoticeable several hours after tocopherol removal. Therefore, induction of exocytosis in combination with therapies requiring endocytic uptake, such as ERT, may represent a new type of drug interaction, yet this strategy could be valuable if properly timed for minimal interference.
U.S. Government work not protected by U.S. copyright.
Figures
Similar articles
-
Lysosomal enzyme delivery by ICAM-1-_targeted nanocarriers bypassing glycosylation- and clathrin-dependent endocytosis.Mol Ther. 2006 Jan;13(1):135-41. doi: 10.1016/j.ymthe.2005.07.687. Epub 2005 Sep 8. Mol Ther. 2006. PMID: 16153895
-
Clathrin-mediated endocytosis is impaired in type A-B Niemann-Pick disease model cells and can be restored by ICAM-1-mediated enzyme replacement.Mol Pharm. 2014 Aug 4;11(8):2887-95. doi: 10.1021/mp500241y. Epub 2014 Jun 26. Mol Pharm. 2014. PMID: 24949999 Free PMC article.
-
Altered Clathrin-Independent Endocytosis in Type A Niemann-Pick Disease Cells and Rescue by ICAM-1-_targeted Enzyme Delivery.Mol Pharm. 2015 May 4;12(5):1366-76. doi: 10.1021/mp5005959. Epub 2015 Apr 23. Mol Pharm. 2015. PMID: 25849869 Free PMC article.
-
The pathogenesis and treatment of acid sphingomyelinase-deficient Niemann-Pick disease.J Inherit Metab Dis. 2007 Oct;30(5):654-63. doi: 10.1007/s10545-007-0632-9. Epub 2007 Jul 12. J Inherit Metab Dis. 2007. PMID: 17632693 Review.
-
Lysosomal enzyme replacement therapies: Historical development, clinical outcomes, and future perspectives.Adv Drug Deliv Rev. 2017 Sep 1;118:109-134. doi: 10.1016/j.addr.2017.05.004. Epub 2017 May 11. Adv Drug Deliv Rev. 2017. PMID: 28502768 Free PMC article. Review.
Cited by
-
Human iPSC-Based Models for the Development of Therapeutics _targeting Neurodegenerative Lysosomal Storage Diseases.Front Mol Biosci. 2020 Sep 18;7:224. doi: 10.3389/fmolb.2020.00224. eCollection 2020. Front Mol Biosci. 2020. PMID: 33062642 Free PMC article. Review.
-
Polymer-based drug delivery systems under investigation for enzyme replacement and other therapies of lysosomal storage disorders.Adv Drug Deliv Rev. 2023 Jun;197:114683. doi: 10.1016/j.addr.2022.114683. Epub 2023 Jan 16. Adv Drug Deliv Rev. 2023. PMID: 36657645 Free PMC article. Review.
-
Altered blood-brain barrier transport of nanotherapeutics in lysosomal storage diseases.J Control Release. 2022 Sep;349:1031-1044. doi: 10.1016/j.jconrel.2022.07.022. Epub 2022 Aug 17. J Control Release. 2022. PMID: 35901858 Free PMC article.
References
-
- Andersen CB, Moestrup SK. (2014) How calcium makes endocytic receptors attractive. Trends Biochem Sci 39:82–90. - PubMed
-
- Ballabio A, Gieselmann V. (2009) Lysosomal disorders: from storage to cellular damage. Biochim Biophys Acta 1793:684–696. - PubMed
-
- Boado RJ, Hui EK, Lu JZ, Zhou QH, Pardridge WM. (2011) Reversal of lysosomal storage in brain of adult MPS-I mice with intravenous Trojan horse-iduronidase fusion protein. Mol Pharm 8:1342–1350. - PubMed
-
- Bradford A, Atkinson J, Fuller N, Rand RP. (2003) The effect of vitamin E on the structure of membrane lipid assemblies. J Lipid Res 44:1940–1945. - PubMed
-
- Cao Q, Zhong XZ, Zou Y, Zhang Z, Toro L, Dong XP. (2015) BK channels alleviate lysosomal storage diseases by providing positive feedback regulation of lysosomal Ca2+ release. Dev Cell 33:427–441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
