Theranostic Nanoparticles for RNA-Based Cancer Treatment
- PMID: 31135134
- PMCID: PMC6701180
- DOI: 10.1021/acs.accounts.9b00101
Theranostic Nanoparticles for RNA-Based Cancer Treatment
Abstract
Certain genetic mutations lead to the development of cancer through unchecked cell growth and division. Cancer is typically treated through surgical resection, radiotherapy, and small-molecule chemotherapy. A relatively recent approach to cancer therapy involves the use of a natural process wherein small RNA molecules regulate gene expression in a pathway known as RNA interference (RNAi). RNA oligomers pair with a network of proteins to form an RNA-induced silencing complex, which inhibits the translation of mRNA into proteins, thereby controlling the expression of gene products. Synthetically produced RNA oligomers may be designed to _target and silence specific oncogenes to provide cancer therapy. The primary challenges facing the use of the RNAi pathway for cancer therapy are the safe and efficacious delivery of RNA payloads and their release at pertinent sites within disease-causing cells. Nucleases are abundant in the bloodstream and intracellular environment, and therapeutic RNA sequences often require a suitable carrier to provide protection from degradation prior to reaching their site of action in the body. The use of metal core nanoparticles (NPs) serving as _targeted delivery vehicles able to shield and direct RNA payloads to their intended destinations have recently gained favor. Biological barriers present in the body establish a size prerequisite for drug delivery vehicles; to overcome recognition by the body's immune system and to gain access to intracellular environments, drug carriers must be small (< 100 nm). Iron oxide and gold core NPs can be synthesized with a high degree of control to create uniform ultrasmall drug delivery vehicles capable of bypassing key biological barriers. While progress is being made in size control of liposomal and polymer NPs, such advances still lag in comparison to the exquisite tunability and time stability of size engineering achievable with metal core NPs at bulk scales. Further, unlike lipid- and viral-based transfection agents, the biodistribution of metal core NPs can be traced using noninvasive imaging techniques that capitalize on the interaction of electromagnetic radiation and the inorganic atoms at the core of the NPs. Finally, metal core NPs have been shown to match the transfection efficiency of conventional RNA-delivery vehicles while also providing less immunogenicity and minimal side effects through the addition of tumor-_targeting ligands on their surface. This Account reviews recent advances in the use of iron oxide and gold NPs for RNAi therapy. An overview of the different types of RNA-based therapies is provided along with a discussion of the advantages and current limitations of the technique. We highlight design considerations for the use of iron oxide and gold NP carriers in RNAi, including a discussion of the importance of size and its role in traversing biological barriers, NP surface modifications required for _targeted delivery and RNA payload release, and auxiliary properties supporting imaging functionality for treatment monitoring. Applications of NPs for combination therapies including the pairing of RNAi with chemotherapy, photothermal therapy, immunotherapy, and radiotherapy are explored through examples. Finally, future perspectives are provided with a focus on the current limitations and the potential for clinical translation of iron oxide and gold NPs in RNAi therapy.
Conflict of interest statement
Notes
The authors declare no competing interests.
Figures

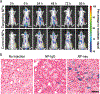
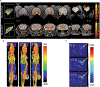


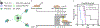
Similar articles
-
Polymer coated gold-ferric oxide superparamagnetic nanoparticles for theranostic applications.J Nanobiotechnology. 2018 Oct 13;16(1):80. doi: 10.1186/s12951-018-0405-7. J Nanobiotechnology. 2018. PMID: 30316298 Free PMC article.
-
Rekindling RNAi Therapy: Materials Design Requirements for In Vivo siRNA Delivery.Adv Mater. 2019 Dec;31(49):e1903637. doi: 10.1002/adma.201903637. Epub 2019 Sep 30. Adv Mater. 2019. PMID: 31566258 Free PMC article. Review.
-
Nanomedicine for cancer diagnosis and therapy: advancement, success and structure-activity relationship.Ther Deliv. 2017 Nov;8(11):1003-1018. doi: 10.4155/tde-2017-0062. Ther Deliv. 2017. PMID: 29061101 Review.
-
Surface engineering of iron oxide nanoparticles for _targeted cancer therapy.Acc Chem Res. 2011 Oct 18;44(10):853-62. doi: 10.1021/ar2000277. Epub 2011 Apr 29. Acc Chem Res. 2011. PMID: 21528865 Free PMC article. Review.
-
Oligonucleotide-based theranostic nanoparticles in cancer therapy.Nanomedicine (Lond). 2016 May;11(10):1287-308. doi: 10.2217/nnm-2016-0035. Epub 2016 Apr 22. Nanomedicine (Lond). 2016. PMID: 27102380 Free PMC article. Review.
Cited by
-
The application of nanoparticles in delivering small RNAs for cancer therapy.Discov Oncol. 2024 Sep 27;15(1):500. doi: 10.1007/s12672-024-01341-1. Discov Oncol. 2024. PMID: 39331172 Free PMC article. Review.
-
Current status and trends in small nucleic acid drug development: Leading the future.Acta Pharm Sin B. 2024 Sep;14(9):3802-3817. doi: 10.1016/j.apsb.2024.05.008. Epub 2024 May 15. Acta Pharm Sin B. 2024. PMID: 39309508 Free PMC article. Review.
-
Late-onset major depressive disorder: exploring the therapeutic potential of enhancing cerebral brain-derived neurotrophic factor expression through _targeted microRNA delivery.Transl Psychiatry. 2024 Sep 3;14(1):352. doi: 10.1038/s41398-024-02935-7. Transl Psychiatry. 2024. PMID: 39227372 Free PMC article. Review.
-
Advances in nucleic acid therapeutics: structures, delivery systems, and future perspectives in cancer treatment.Clin Exp Med. 2024 Aug 28;24(1):200. doi: 10.1007/s10238-024-01463-4. Clin Exp Med. 2024. PMID: 39196428 Free PMC article. Review.
-
_targeted Delivery Strategies for Multiple Myeloma and Their Adverse Drug Reactions.Pharmaceuticals (Basel). 2024 Jun 25;17(7):832. doi: 10.3390/ph17070832. Pharmaceuticals (Basel). 2024. PMID: 39065683 Free PMC article. Review.
References
-
- Sen GL; Blau HM A brief history of RNAi: the silence of the genes. FASEB J 2006, 20, 1293–1299. - PubMed
-
- Iwasaki S; Tomari Y: Reconstitution of RNA Interference Machinery. In Argonaute Proteins: Methods and Protocols; Okamura K, Nakanishi K, Eds.; Methods in Molecular Biology; Humana Press: New York, NY, 2018; Vol. 1680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

