Specialized coding of sensory, motor and cognitive variables in VTA dopamine neurons
- PMID: 31142844
- PMCID: PMC7147811
- DOI: 10.1038/s41586-019-1261-9
Specialized coding of sensory, motor and cognitive variables in VTA dopamine neurons
Abstract
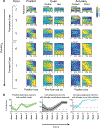
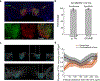
There is increased appreciation that dopamine neurons in the midbrain respond not only to reward1 and reward-predicting cues1,2, but also to other variables such as the distance to reward3, movements4-9 and behavioural choices10,11. An important question is how the responses to these diverse variables are organized across the population of dopamine neurons. Whether individual dopamine neurons multiplex several variables, or whether there are subsets of neurons that are specialized in encoding specific behavioural variables remains unclear. This fundamental question has been difficult to resolve because recordings from large populations of individual dopamine neurons have not been performed in a behavioural task with sufficient complexity to examine these diverse variables simultaneously. Here, to address this gap, we used two-photon calcium imaging through an implanted lens to record the activity of more than 300 dopamine neurons from the ventral tegmental area of the mouse midbrain during a complex decision-making task. As mice navigated in a virtual-reality environment, dopamine neurons encoded an array of sensory, motor and cognitive variables. These responses were functionally clustered, such that subpopulations of neurons transmitted information about a subset of behavioural variables, in addition to encoding reward. These functional clusters were spatially organized, with neighbouring neurons more likely to be part of the same cluster. Together with the topography between dopamine neurons and their projections, this specialization and anatomical organization may aid downstream circuits in correctly interpreting the wide range of signals transmitted by dopamine neurons.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures








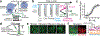


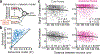

Similar articles
-
Context-Dependent Multiplexing by Individual VTA Dopamine Neurons.J Neurosci. 2020 Sep 23;40(39):7489-7509. doi: 10.1523/JNEUROSCI.0502-20.2020. Epub 2020 Aug 28. J Neurosci. 2020. PMID: 32859713 Free PMC article.
-
Synergy of Distinct Dopamine Projection Populations in Behavioral Reinforcement.Neuron. 2020 Mar 4;105(5):909-920.e5. doi: 10.1016/j.neuron.2019.11.024. Epub 2019 Dec 23. Neuron. 2020. PMID: 31879163 Free PMC article.
-
Activation of Pedunculopontine Glutamate Neurons Is Reinforcing.J Neurosci. 2017 Jan 4;37(1):38-46. doi: 10.1523/JNEUROSCI.3082-16.2016. J Neurosci. 2017. PMID: 28053028 Free PMC article.
-
Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat: Historical perspective.Brain Res. 2016 Aug 15;1645:68-70. doi: 10.1016/j.brainres.2015.12.041. Epub 2015 Dec 28. Brain Res. 2016. PMID: 26731335 Review.
-
VTA GABA Neurons at the Interface of Stress and Reward.Front Neural Circuits. 2019 Dec 5;13:78. doi: 10.3389/fncir.2019.00078. eCollection 2019. Front Neural Circuits. 2019. PMID: 31866835 Free PMC article. Review.
Cited by
-
Frustrative Nonreward: Behavior, Circuits, Neurochemistry, and Disorders.J Neurosci. 2024 Oct 2;44(40):e1021242024. doi: 10.1523/JNEUROSCI.1021-24.2024. J Neurosci. 2024. PMID: 39358023 Review.
-
Multidimensional encoding of movement and contextual variables by rat globus pallidus neurons during a novel environment exposure task.iScience. 2022 Aug 28;25(9):105024. doi: 10.1016/j.isci.2022.105024. eCollection 2022 Sep 16. iScience. 2022. PMID: 36117990 Free PMC article.
-
Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control.Cells. 2021 Mar 26;10(4):735. doi: 10.3390/cells10040735. Cells. 2021. PMID: 33810328 Free PMC article. Review.
-
Single neuron responses to perceptual difficulty in the mouse auditory cortex.Sci Adv. 2024 Aug 16;10(33):eadp9816. doi: 10.1126/sciadv.adp9816. Epub 2024 Aug 14. Sci Adv. 2024. PMID: 39141740 Free PMC article.
-
Selecting and Executing Actions for Rewards.J Neurosci. 2020 Aug 19;40(34):6474-6476. doi: 10.1523/JNEUROSCI.1250-20.2020. J Neurosci. 2020. PMID: 32817389 Free PMC article. No abstract available.
References
-
- Schultz W, Dayan P & Montague PR A Neural Substrate of Prediction and Reward. Science 275, 1593–1599 (1997). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

