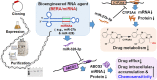
Bioengineered miR-27b-3p and miR-328-3p modulate drug metabolism and disposition via the regulation of _target ADME gene expression
- PMID: 31193825
- PMCID: PMC6543075
- DOI: 10.1016/j.apsb.2018.12.002
Bioengineered miR-27b-3p and miR-328-3p modulate drug metabolism and disposition via the regulation of _target ADME gene expression
Abstract
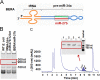
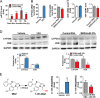
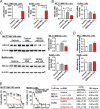
Drug-metabolizing enzymes, transporters, and nuclear receptors are essential for the absorption, distribution, metabolism, and excretion (ADME) of drugs and xenobiotics. MicroRNAs participate in the regulation of ADME gene expression via imperfect complementary Watson-Crick base pairings with _target transcripts. We have previously reported that Cytochrome P450 3A4 (CYP3A4) and ATP-binding cassette sub-family G member 2 (ABCG2) are regulated by miR-27b-3p and miR-328-3p, respectively. Here we employed our newly established RNA bioengineering technology to produce bioengineered RNA agents (BERA), namely BERA/miR-27b-3p and BERA/miR-328-3p, via fermentation. When introduced into human cells, BERA/miR-27b-3p and BERA/miR-328-3p were selectively processed to _target miRNAs and thus knock down CYP3A4 and ABCG2 mRNA and their protein levels, respectively, as compared to cells treated with vehicle or control RNA. Consequently, BERA/miR-27b-3p led to a lower midazolam 1'-hydroxylase activity, indicating the reduction of CYP3A4 activity. Likewise, BERA/miR-328-3p treatment elevated the intracellular accumulation of anticancer drug mitoxantrone, a classic substrate of ABCG2, hence sensitized the cells to chemotherapy. The results indicate that biologic miRNA agents made by RNA biotechnology may be applied to research on miRNA functions in the regulation of drug metabolism and disposition that could provide insights into the development of more effective therapies.
Keywords: 3′-UTR, 3′-untranslated region;, VDR, vitamin D receptor; ABCG2; ABCG2, ATP-binding cassette sub-family G member 2;, ADME, absorption, distribution, metabolism, and excretion; BERA, bioengineered RNA agent;, CYP, cytochrome P450; Bioengineered RNA; CYP3A4; Drug disposition; E. coli, Escherichia coli;, FPLC, fast protein liquid chromatography; LC--MS/MS, liquid chromatographytandem mass spectroscopy;, microRNA, miR or miRNA; RNAi, RNA interference;, RT-qPCR, reverse transcription quantitative real-time polymerase chain reaction; RXRα, retinoid X receptor α;, tRNA, transfer RNA; miR-27b; miR-328; ncRNA, noncoding RNA;, PAGE, polyacrylamide gel electrophoresis.
Figures
Similar articles
-
Recombinant Technologies Facilitate Drug Metabolism, Pharmacokinetics, and General Biomedical Research.Drug Metab Dispos. 2023 Jun;51(6):685-699. doi: 10.1124/dmd.122.001008. Epub 2023 Mar 22. Drug Metab Dispos. 2023. PMID: 36948592 Free PMC article. Review.
-
Bioengineered miR-328-3p modulates GLUT1-mediated glucose uptake and metabolism to exert synergistic antiproliferative effects with chemotherapeutics.Acta Pharm Sin B. 2020 Jan;10(1):159-170. doi: 10.1016/j.apsb.2019.11.001. Epub 2019 Nov 7. Acta Pharm Sin B. 2020. PMID: 31993313 Free PMC article.
-
Rapid production of novel pre-microRNA agent hsa-mir-27b in Escherichia coli using recombinant RNA technology for functional studies in mammalian cells.Drug Metab Dispos. 2014 Nov;42(11):1791-5. doi: 10.1124/dmd.114.060145. Epub 2014 Aug 26. Drug Metab Dispos. 2014. PMID: 25161167 Free PMC article.
-
Single bioengineered ncRNA molecule for dual-_targeting toward the control of non-small cell lung cancer patient-derived xenograft tumor growth.Biochem Pharmacol. 2021 Jul;189:114392. doi: 10.1016/j.bcp.2020.114392. Epub 2021 Jan 3. Biochem Pharmacol. 2021. PMID: 33359565 Free PMC article.
-
Recent Advances in Novel Recombinant RNAs for Studying Post-transcriptional Gene Regulation in Drug Metabolism and Disposition.Curr Drug Metab. 2023;24(3):175-189. doi: 10.2174/1389200224666230425232433. Curr Drug Metab. 2023. PMID: 37170982 Free PMC article. Review.
Cited by
-
MicroRNA-Based Combinatorial Cancer Therapy: Effects of MicroRNAs on the Efficacy of Anti-Cancer Therapies.Cells. 2019 Dec 20;9(1):29. doi: 10.3390/cells9010029. Cells. 2019. PMID: 31861937 Free PMC article. Review.
-
Recombinant Technologies Facilitate Drug Metabolism, Pharmacokinetics, and General Biomedical Research.Drug Metab Dispos. 2023 Jun;51(6):685-699. doi: 10.1124/dmd.122.001008. Epub 2023 Mar 22. Drug Metab Dispos. 2023. PMID: 36948592 Free PMC article. Review.
-
Autophagy and beyond: Unraveling the complexity of UNC-51-like kinase 1 (ULK1) from biological functions to therapeutic implications.Acta Pharm Sin B. 2022 Oct;12(10):3743-3782. doi: 10.1016/j.apsb.2022.06.004. Epub 2022 Jun 11. Acta Pharm Sin B. 2022. PMID: 36213540 Free PMC article. Review.
-
Current trends in drug metabolism and pharmacokinetics.Acta Pharm Sin B. 2019 Nov;9(6):1113-1144. doi: 10.1016/j.apsb.2019.10.001. Epub 2019 Oct 18. Acta Pharm Sin B. 2019. PMID: 31867160 Free PMC article. Review.
-
VARIDT 1.0: variability of drug transporter database.Nucleic Acids Res. 2020 Jan 8;48(D1):D1042-D1050. doi: 10.1093/nar/gkz779. Nucleic Acids Res. 2020. PMID: 31495872 Free PMC article.
References
-
- Lu A.Y. Drug-metabolism research challenges in the new millennium: individual variability in drug therapy and drug safety. Drug Metab Dispos. 1998;26:1217–1222. - PubMed
-
- Zanger U.M., Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103–141. - PubMed
-
- Manikandan P., Nagini S. Cytochrome P450 structure, function and clinical significance: a review. Curr Drug _targets. 2018;19:38–54. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

