Cross-talk between Human Spinal Cord μ-opioid Receptor 1Y Isoform and Gastrin-releasing Peptide Receptor Mediates Opioid-induced Scratching Behavior
- PMID: 31314749
- PMCID: PMC7098053
- DOI: 10.1097/ALN.0000000000002776
Cross-talk between Human Spinal Cord μ-opioid Receptor 1Y Isoform and Gastrin-releasing Peptide Receptor Mediates Opioid-induced Scratching Behavior
Abstract
Background: Although spinal opioids are safe and effective, pruritus is common and distressing. The authors previously demonstrated in mouse spinal cord that interactions between μ-opioid receptor isoform 1D and gastrin releasing peptide receptor mediate morphine-induced scratch. The C-terminal of 1D inhibits morphine-induced scratch without affecting analgesia. The authors hypothesize that human spinal cord also contains itch-specific μ-opioid receptor isoforms which interact with gastrin releasing peptide receptor.
Methods: Reverse transcription polymerase chain reaction was performed on human spinal cord complimentary DNA from two human cadavers. Calcium responses to morphine (1 μM) were examined using calcium imaging microscopy on human cells (HEK293) coexpressing gastrin releasing peptide receptor and different human μ-opioid receptor isoforms. The authors assessed morphine-induced scratching behavior and thermal analgesia in mice following intrathecal injection of morphine (0.3 nmol) and a transactivator of transcription peptide designed from C-terminal sequences of 1Y isoform (0, 0.1, and 0.4 nmol).
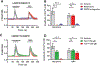
Results: The authors demonstrated 1Y expression in the spinal cord dorsal horn. Morphine administration evoked a calcium response (mean ± SD) (57 ± 13 nM) in cells coexpressing both gastrin releasing peptide receptor and the 1Y isomer. This was blocked by 10 μM naltrexone (0.7 ± 0.4 nM; P < 0.0001), 1 μM gastrin-releasing peptide receptor antagonist (3 ± 2 nM; P < 0.0001), or 200 μM 1Y-peptide (2 + 2 nM; P < 0.0001). In mice, 0.4 nmol 1Y-peptide significantly attenuated morphine-induced scratching behaviors (scratching bouts, vehicle vs. 1Y-peptide) (92 ± 31 vs. 38 ± 29; P = 0.011; n = 6 to 7 mice per group), without affecting morphine antinociception in warm water tail immersion test (% of maximum possible effect) (70 ± 21 vs. 67 ± 22; P = 0.80; n = 6 mice per group).
Conclusions: Human μ-opioid receptor 1Y isomer is a C-terminal splicing variant of Oprm1 gene identified in human spinal cord. Cross-talk between 1Y and gastrin releasing peptide receptor is required for mediating opioid-induced pruritus. Disrupting the cross talk may have implications for therapeutic uncoupling of desired analgesic effects from side effects of opioids.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures


Comment in
-
Non-Peer-reviewed Preprint Articles as References in Anesthesiology: Concerns.Anesthesiology. 2021 May 1;134(5):820. doi: 10.1097/ALN.0000000000003714. Anesthesiology. 2021. PMID: 33592106 No abstract available.
Similar articles
-
Central opioid receptors mediate morphine-induced itch and chronic itch via disinhibition.Brain. 2021 Mar 3;144(2):665-681. doi: 10.1093/brain/awaa430. Brain. 2021. PMID: 33367648
-
Physiological function of gastrin-releasing peptide and neuromedin B receptors in regulating itch scratching behavior in the spinal cord of mice.PLoS One. 2013 Jun 24;8(6):e67422. doi: 10.1371/journal.pone.0067422. Print 2013. PLoS One. 2013. PMID: 23826298 Free PMC article.
-
The role of central mu opioid receptors in opioid-induced itch in primates.J Pharmacol Exp Ther. 2004 Jul;310(1):169-76. doi: 10.1124/jpet.103.061101. Epub 2004 Mar 25. J Pharmacol Exp Ther. 2004. PMID: 15044556
-
Role of spinal neurotransmitter receptors in itch: new insights into therapies and drug development.CNS Neurosci Ther. 2011 Dec;17(6):742-9. doi: 10.1111/j.1755-5949.2010.00201.x. Epub 2010 Oct 15. CNS Neurosci Ther. 2011. PMID: 20950328 Free PMC article. Review.
-
Neural processing of itch.Neuroscience. 2013 Oct 10;250:697-714. doi: 10.1016/j.neuroscience.2013.07.035. Epub 2013 Jul 24. Neuroscience. 2013. PMID: 23891755 Free PMC article. Review.
Cited by
-
Retrospectively assessed subjective effects of initial opioid use differ between opioid misusers with opioid use disorder (OUD) and those who never progressed to OUD: Data from a pilot and a replication sample.J Neurosci Res. 2022 Jan;100(1):353-361. doi: 10.1002/jnr.24643. Epub 2020 May 28. J Neurosci Res. 2022. PMID: 32468677 Free PMC article.
-
BNP facilitates NMB-encoded histaminergic itch via NPRC-NMBR crosstalk.Elife. 2021 Dec 17;10:e71689. doi: 10.7554/eLife.71689. Elife. 2021. PMID: 34919054 Free PMC article.
-
Advances in Understanding the Initial Steps of Pruritoceptive Itch: How the Itch Hits the Switch.Int J Mol Sci. 2020 Jul 10;21(14):4883. doi: 10.3390/ijms21144883. Int J Mol Sci. 2020. PMID: 32664385 Free PMC article. Review.
-
Characterization of the expression of gastrin-releasing peptide and its receptor in the trigeminal and spinal somatosensory systems of Japanese macaque monkeys: Insight into humans.J Comp Neurol. 2022 Nov;530(16):2804-2819. doi: 10.1002/cne.25376. Epub 2022 Jun 10. J Comp Neurol. 2022. PMID: 35686563 Free PMC article.
-
A neuropeptide code for itch.Nat Rev Neurosci. 2021 Dec;22(12):758-776. doi: 10.1038/s41583-021-00526-9. Epub 2021 Oct 18. Nat Rev Neurosci. 2021. PMID: 34663954 Free PMC article. Review.
References
-
- Behar M, Magora F, Olshwang D, Davidson JT: Epidural morphine in treatment of pain. Lancet 1979; 1:527–9 - PubMed
-
- Eltzschig HK, Lieberman ES, Camann WR: Regional anesthesia and analgesia for labor and delivery. N Engl J Med 2003; 348:319–32 - PubMed
-
- Carvalho B, Wang P, Cohen SE: A survey of labor patient-controlled epidural anesthesia practice in California hospitals. Int J Obstet Anesth 2006; 15:217–22 - PubMed
-
- Practice Guidelines for Obstetric Anesthesia: An Updated Report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia and the Society for Obstetric Anesthesia and Perinatology. Anesthesiology 2016; 124: 270–300 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

