Recruitment and Activation of the ULK1/Atg1 Kinase Complex in Selective Autophagy
- PMID: 31351898
- PMCID: PMC6971721
- DOI: 10.1016/j.jmb.2019.07.027
Recruitment and Activation of the ULK1/Atg1 Kinase Complex in Selective Autophagy
Abstract
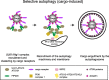
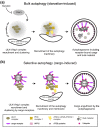
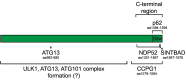
Autophagy is a major cellular degradation pathway, which mediates the delivery of cytoplasmic cargo material into lysosomes. This is achieved by the specific sequestration of the cargo within double-membrane vesicles, the autophagosomes, which form de novo around this material. Autophagosome formation requires the action of a conserved set of factors, which act in hierarchical manner. The ULK1/Atg1 kinase complex is one of the most upstream acting components of the autophagy machinery. Here we discuss recent insights into the mechanisms of ULK1/Atg1 recruitment and activation at the cargo during selective autophagy. In particular, we will focus on the role of cargo receptors such as p62 and NDP52 during this process and discuss the emerging concept that cargo receptors act upstream of the autophagy machinery during cargo-induced selective autophagy.
Keywords: autophagosome; cargo receptor; protein kinase; quality control; signaling.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

Similar articles
-
Aberrant autophagosome formation occurs upon small molecule inhibition of ULK1 kinase activity.Life Sci Alliance. 2020 Oct 27;3(12):e202000815. doi: 10.26508/lsa.202000815. Print 2020 Dec. Life Sci Alliance. 2020. PMID: 33109685 Free PMC article.
-
Regulation of Autophagy By Signaling Through the Atg1/ULK1 Complex.J Mol Biol. 2016 May 8;428(9 Pt A):1725-41. doi: 10.1016/j.jmb.2016.03.030. Epub 2016 Apr 6. J Mol Biol. 2016. PMID: 27059781 Review.
-
Scaffold proteins in bulk and selective autophagy.Prog Mol Biol Transl Sci. 2020;172:15-35. doi: 10.1016/bs.pmbts.2020.01.009. Epub 2020 Mar 6. Prog Mol Biol Transl Sci. 2020. PMID: 32620241 Review.
-
Multilayered regulation of autophagy by the Atg1 kinase orchestrates spatial and temporal control of autophagosome formation.Mol Cell. 2021 Dec 16;81(24):5066-5081.e10. doi: 10.1016/j.molcel.2021.10.024. Epub 2021 Nov 18. Mol Cell. 2021. PMID: 34798055 Free PMC article.
-
Two Independent Pathways within Selective Autophagy Converge to Activate Atg1 Kinase at the Vacuole.Mol Cell. 2016 Oct 20;64(2):221-235. doi: 10.1016/j.molcel.2016.09.008. Mol Cell. 2016. PMID: 27768871
Cited by
-
Recent advances in the understanding of autophagosome biogenesis.F1000Res. 2020 Mar 26;9:F1000 Faculty Rev-212. doi: 10.12688/f1000research.22111.1. eCollection 2020. F1000Res. 2020. PMID: 32266060 Free PMC article. Review.
-
Activation and _targeting of ATG8 protein lipidation.Cell Discov. 2020 May 5;6:23. doi: 10.1038/s41421-020-0155-1. eCollection 2020. Cell Discov. 2020. PMID: 32377373 Free PMC article. Review.
-
Protective or Harmful: The Dual Roles of Autophagy in Diabetic Retinopathy.Front Med (Lausanne). 2021 Mar 25;8:644121. doi: 10.3389/fmed.2021.644121. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33842506 Free PMC article. Review.
-
LC3-associated endocytosis and the functions of Rubicon and ATG16L1.Sci Adv. 2022 Oct 28;8(43):eabo5600. doi: 10.1126/sciadv.abo5600. Epub 2022 Oct 26. Sci Adv. 2022. PMID: 36288306 Free PMC article. Review.
-
The ubiquitin E2 enzyme UBE2QL1 mediates lysophagy.EMBO Rep. 2019 Oct 4;20(10):e49104. doi: 10.15252/embr.201949104. Epub 2019 Sep 15. EMBO Rep. 2019. PMID: 31523916 Free PMC article.
References
-
- Blommaart E.F.C., Luiken J.J.F.P., Blommaart P.J.E., van Woerkom G.M., Meijer A.J. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. Journal of Biological Chemistry. 1995;270:2320–2326. - PubMed
-
- Noda T., Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. Journal of Biological Chemistry. 1998;273:3963–3966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

