Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS?
- PMID: 31382355
- PMCID: PMC6696313
- DOI: 10.3390/ijms20153775
Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS?
Abstract
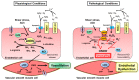
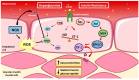
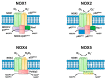
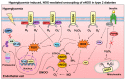
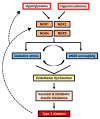
NADPH oxidases (NOX) are enzyme complexes that have received much attention as key molecules in the development of vascular dysfunction. NOX have the primary function of generating reactive oxygen species (ROS), and are considered the main source of ROS production in endothelial cells. The endothelium is a thin monolayer that lines the inner surface of blood vessels, acting as a secretory organ to maintain homeostasis of blood flow. The enzymatic production of nitric oxide (NO) by endothelial NO synthase (eNOS) is critical in mediating endothelial function, and oxidative stress can cause dysregulation of eNOS and endothelial dysfunction. Insulin is a stimulus for increases in blood flow and endothelium-dependent vasodilation. However, cardiovascular disease and type 2 diabetes are characterized by poor control of the endothelial cell redox environment, with a shift toward overproduction of ROS by NOX. Studies in models of type 2 diabetes demonstrate that aberrant NOX activation contributes to uncoupling of eNOS and endothelial dysfunction. It is well-established that endothelial dysfunction precedes the onset of cardiovascular disease, therefore NOX are important molecular links between type 2 diabetes and vascular complications. The aim of the current review is to describe the normal, healthy physiological mechanisms involved in endothelial function, and highlight the central role of NOX in mediating endothelial dysfunction when glucose homeostasis is impaired.
Keywords: NADPH oxidase; ROS; eNOS; endothelium; glucose; hyperglycemia; insulin resistance; obesity; reactive oxygen species; type 2 diabetes; vascular function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The p47phox- and NADPH oxidase organiser 1 (NOXO1)-dependent activation of NADPH oxidase 1 (NOX1) mediates endothelial nitric oxide synthase (eNOS) uncoupling and endothelial dysfunction in a streptozotocin-induced murine model of diabetes.Diabetologia. 2012 Jul;55(7):2069-79. doi: 10.1007/s00125-012-2557-6. Epub 2012 May 2. Diabetologia. 2012. PMID: 22549734 Free PMC article.
-
β3 Adrenergic Stimulation Restores Nitric Oxide/Redox Balance and Enhances Endothelial Function in Hyperglycemia.J Am Heart Assoc. 2016 Feb 19;5(2):e002824. doi: 10.1161/JAHA.115.002824. J Am Heart Assoc. 2016. PMID: 26896479 Free PMC article.
-
Role of eNOS- and NOX-containing microparticles in endothelial dysfunction in patients with obesity.Obesity (Silver Spring). 2016 Jun;24(6):1305-12. doi: 10.1002/oby.21508. Epub 2016 Apr 30. Obesity (Silver Spring). 2016. PMID: 27130266
-
Reactive oxygen species and endothelial function--role of nitric oxide synthase uncoupling and Nox family nicotinamide adenine dinucleotide phosphate oxidases.Basic Clin Pharmacol Toxicol. 2012 Jan;110(1):87-94. doi: 10.1111/j.1742-7843.2011.00785.x. Epub 2011 Sep 28. Basic Clin Pharmacol Toxicol. 2012. PMID: 21883939 Review.
-
Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and NADPH oxidase.Can J Physiol Pharmacol. 2010 Mar;88(3):241-8. doi: 10.1139/Y10-018. Can J Physiol Pharmacol. 2010. PMID: 20393589 Review.
Cited by
-
Cellular Senescence as the Pathogenic Hub of Diabetes-Related Wound Chronicity.Front Endocrinol (Lausanne). 2020 Sep 16;11:573032. doi: 10.3389/fendo.2020.573032. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33042026 Free PMC article. Review.
-
The Intrinsic Virtues of EGCG, an Extremely Good Cell Guardian, on Prevention and Treatment of Diabesity Complications.Molecules. 2020 Jul 4;25(13):3061. doi: 10.3390/molecules25133061. Molecules. 2020. PMID: 32635492 Free PMC article. Review.
-
Protective effect of ellagic acid against high-glucose-induced injury in human umbilical venous endothelial cells.Avicenna J Phytomed. 2024 Jan-Feb;14(1):138-141. doi: 10.22038/AJP.2023.22910. Avicenna J Phytomed. 2024. PMID: 38948172 Free PMC article.
-
Metabolic memory: mechanisms and diseases.Signal Transduct _target Ther. 2024 Feb 28;9(1):38. doi: 10.1038/s41392-024-01755-x. Signal Transduct _target Ther. 2024. PMID: 38413567 Free PMC article. Review.
-
Pediatric Type 1 Diabetes: Mechanisms and Impact of Technologies on Comorbidities and Life Expectancy.Int J Mol Sci. 2023 Jul 26;24(15):11980. doi: 10.3390/ijms241511980. Int J Mol Sci. 2023. PMID: 37569354 Free PMC article. Review.
References
-
- Kahn S.E., Prigeon R.L., McCulloch D.K., Boyko E.J., Bergman R.N., Schwartz M.W., Neifing J.L., Ward W.K., Beard J.C., Palmer J.P. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects: Evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. doi: 10.2337/diab.42.11.1663. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

