Adoption of New Glucose-Lowering Medications in the U.S.-The Case of SGLT2 Inhibitors: Nationwide Cohort Study
- PMID: 31418588
- PMCID: PMC7207017
- DOI: 10.1089/dia.2019.0213
Adoption of New Glucose-Lowering Medications in the U.S.-The Case of SGLT2 Inhibitors: Nationwide Cohort Study
Abstract
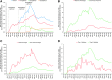
Background: High-quality diabetes care is evidence-based, timely, and equitable. Sodium-glucose cotransporter-2 inhibitors (SGLT2i) are the most recently approved class of glucose-lowering medications with additional cardio- and renal-protective benefits and low risk of hypoglycemia. Cardiovascular and kidney disease are among the most common chronic diabetes complications, whereas hypoglycemia is the most prevalent adverse effect of glucose-lowering therapy. We examine the sociodemographic and clinical factors associated with early SGLT2i initiation and appropriateness of use based on contemporaneous scientific evidence. Materials and Methods: Retrospective analysis of medical and pharmacy claims data from OptumLabs® Data Warehouse for commercially insured and Medicare Advantage adult beneficiaries with diabetes types 1 and 2, who filled any glucose-lowering medication between January 1, 2013 and December 31, 2016. Demographic (age, sex, race, income), clinical (comorbidities), and insurance-related factors affecting first prescription for a SGLT2i were examined using multivariable logistic regression. Results: Among 1,054,727 adults with pharmacologically treated diabetes, 7.2% (n = 75,500) initiated a SGLT2i. Patients with prior myocardial infarction (MI) (odds ratio [OR]: 0.94, 95% confidence interval [CI]: 0.91-0.96), heart failure (HF) (OR: 0.93, 95% CI: 0.91-0.94), kidney disease (OR: 0.80, 95% CI: 0.78-0.81), and severe hypoglycemia (OR: 0.96, 95% CI: 0.94-0.98) were all less likely to start a SGLT2i; P < 0.001 for all. SGLT2i were also less likely to be started by patients ≥75 years (OR: 0.57, 95% CI: 0.55-0.59, vs. 18-44 years), Black patients (OR: 0.93, 95% CI: 0.91-0.95, vs. White), and those with Medicare Advantage insurance (OR: 0.63, 95% CI: 0.62-0.64, vs. commercial). Conclusions: Younger, healthier, non-Black patients with commercial health insurance were most likely to start taking SGLT2i. Patients with MI, HF, kidney disease, and prior hypoglycemia were less likely to use SGLT2i, despite evidence supporting their preferential use in these patients. Efforts to address this treatment-risk paradox may help improve health outcomes among patients with type 2 diabetes.
Keywords: Administrative claims data; Diabetes mellitus; Evidence-based medicine; Health services research; Pharmacoepidemiology; SGLT2 inhibitor.
Conflict of interest statement
No competing financial interests exist.
Figures
Similar articles
-
Comparison of Diabetes Medications Used by Adults With Commercial Insurance vs Medicare Advantage, 2016 to 2019.JAMA Netw Open. 2021 Feb 1;4(2):e2035792. doi: 10.1001/jamanetworkopen.2020.35792. JAMA Netw Open. 2021. PMID: 33523188 Free PMC article.
-
Demographic and Clinical Profiles of Type 2 Diabetes Mellitus Patients Initiating Canagliflozin Versus DPP-4 Inhibitors in a Large U.S. Managed Care Population.J Manag Care Spec Pharm. 2015 Dec;21(12):1204-12. doi: 10.18553/jmcp.2015.21.12.1204. J Manag Care Spec Pharm. 2015. PMID: 26679969 Free PMC article.
-
Sodium-glucose cotransporter 2 inhibitors vs. sitagliptin in heart failure and type 2 diabetes: an observational cohort study.Eur Heart J. 2023 Jun 25;44(24):2216-2230. doi: 10.1093/eurheartj/ehad273. Eur Heart J. 2023. PMID: 37259575 Free PMC article.
-
SODIUM GLUCOSE COTRANSPORTER 2 AND DIPEPTIDYL PEPTIDASE-4 INHIBITION: PROMISE OF A DYNAMIC DUO.Endocr Pract. 2017 Jul;23(7):831-840. doi: 10.4158/EP161725.RA. Epub 2017 Mar 23. Endocr Pract. 2017. PMID: 28332871 Review.
-
The Role of Sodium-Glucose Co-Transporter 2 Inhibitors in the Treatment of Type 2 Diabetes.Clin Ther. 2015 Jun 1;37(6):1150-66. doi: 10.1016/j.clinthera.2015.03.004. Epub 2015 Apr 16. Clin Ther. 2015. PMID: 25891804 Review.
Cited by
-
Evaluating the appropriateness and the factors associated with sodium-glucose co-transporter 2 inhibitors prescribing in a Middle Eastern country: a cross-sectional study.Int J Clin Pharm. 2024 Nov 21. doi: 10.1007/s11096-024-01828-5. Online ahead of print. Int J Clin Pharm. 2024. PMID: 39570571
-
Effects of SGLT2 Inhibitors on Cardiac Mechanics in Hispanic and Black Diabetic Patients.J Clin Med. 2024 Aug 4;13(15):4555. doi: 10.3390/jcm13154555. J Clin Med. 2024. PMID: 39124821 Free PMC article.
-
Trends in prognosis and use of SGLT2i and GLP-1 RA in patients with diabetes and coronary artery disease.Cardiovasc Diabetol. 2024 Aug 7;23(1):290. doi: 10.1186/s12933-024-02365-1. Cardiovasc Diabetol. 2024. PMID: 39113013 Free PMC article.
-
Disparities in Use of Novel Diabetes Medications by Insurance: A Nationally Representative Cohort Study.J Gen Intern Med. 2024 Nov;39(15):2987-2994. doi: 10.1007/s11606-024-08961-x. Epub 2024 Jul 31. J Gen Intern Med. 2024. PMID: 39085578 Free PMC article.
-
REPRESENTATION AND EXTRAPOLATION: EVIDENCE FROM CLINICAL TRIALS.Q J Econ. 2024 Feb;139(1):575-635. doi: 10.1093/qje/qjad036. Epub 2023 Sep 5. Q J Econ. 2024. PMID: 38859982 Free PMC article.
References
-
- CDC. Centers for Disease Control and Prevention: National Diabetes Statistics Report, 2017. Centers for Disease Control and Prevention US Department of Health and Human Services, updated February 24, 2018. https://www.cdc.gov/diabetes/data/statistics/statistics-report.html (accessed July5, 2019)
-
- ADA. American Diabetes Association: Section 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2019. Diabetes Care 2019;42:S34–S45 - PubMed
-
- Zinman B, Wanner C, Lachin JM, et al. : Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 - PubMed
-
- Wanner C, Inzucchi SE, Lachin JM, et al. : Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334 - PubMed
-
- Neal B, Perkovic V, Mahaffey KW, et al. : Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

