Influenza Vaccination Primes Human Myeloid Cell Cytokine Secretion and NK Cell Function
- PMID: 31427444
- PMCID: PMC6739223
- DOI: 10.4049/jimmunol.1801648
Influenza Vaccination Primes Human Myeloid Cell Cytokine Secretion and NK Cell Function
Abstract
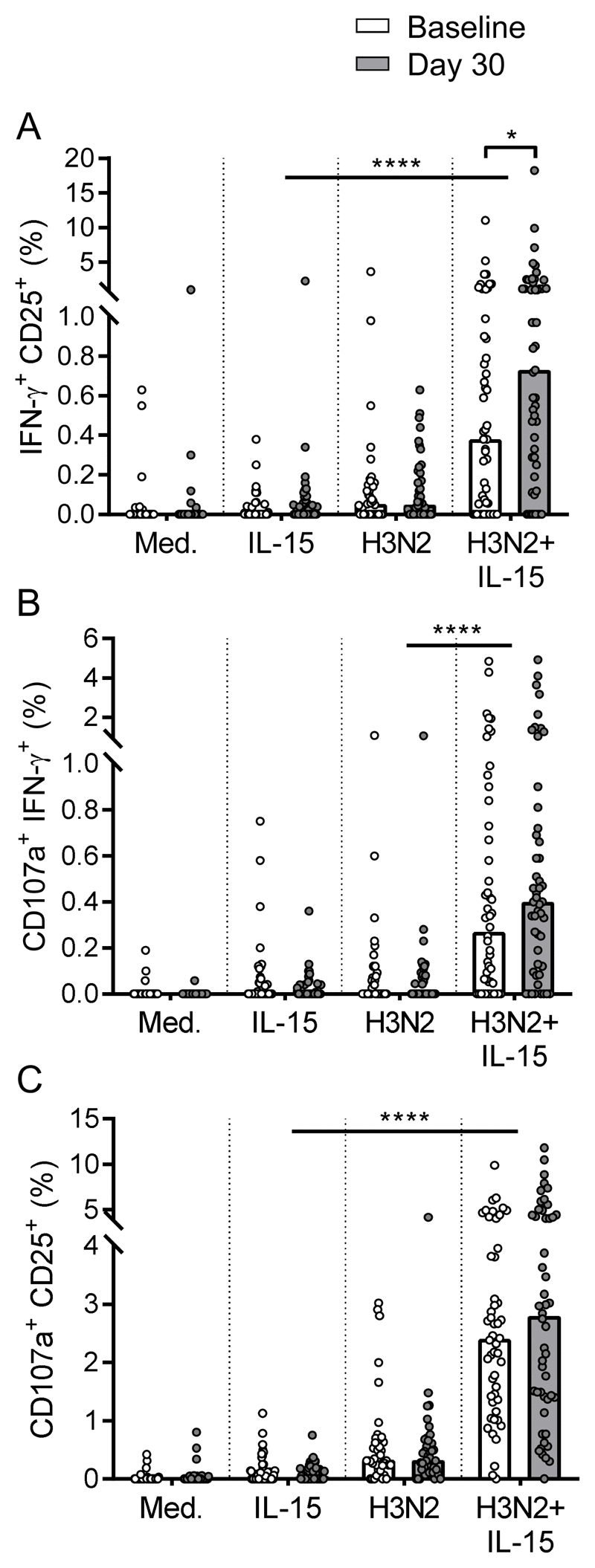
Cytokine-induced memory-like (CIML) NK cells generated in response to proinflammatory cytokines in vitro and in vivo can also be generated by vaccination, exhibiting heightened responses to cytokine stimulation months after their initial induction. Our previous study demonstrated that in vitro human NK cell responses to inactivated influenza virus were also indirectly augmented by very low doses of IL-15, which increased induction of myeloid cell-derived cytokine secretion. These findings led us to hypothesize that IL-15 stimulation could reveal a similar effect for active influenza vaccination and influence CIML NK cell effector functions. In this study, 51 healthy adults were vaccinated with seasonal influenza vaccine, and PBMC were collected before and up to 30 d after vaccination. Myeloid and lymphoid cell cytokine secretion was measured after in vitro PBMC restimulation with low-dose IL-15, alone or in combination with inactivated H3N2 virus; the associated NK cell response was assessed by flow cytometry. PBMC collected 30 d postvaccination showed heightened cytokine production in response to IL-15 compared with PBMC collected at baseline; these responses were further enhanced when IL-15 was combined with H3N2. NK cell activation in response to IL-15 alone (CD25) and H3N2 plus IL-15 (CD25 and IFN-γ) was enhanced postvaccination. We also observed proliferation of less-differentiated NK cells with downregulation of cytokine receptors as early as 3 d after vaccination, suggesting cytokine stimulation in vivo. We conclude that vaccination-induced "training" of accessory cells combines with the generation of CIML NK cells to enhance the overall NK cell response postvaccination.
Copyright © 2019 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Influenza Vaccination Generates Cytokine-Induced Memory-like NK Cells: Impact of Human Cytomegalovirus Infection.J Immunol. 2016 Jul 1;197(1):313-25. doi: 10.4049/jimmunol.1502049. Epub 2016 May 27. J Immunol. 2016. PMID: 27233958 Free PMC article.
-
IL-15 Promotes Polyfunctional NK Cell Responses to Influenza by Boosting IL-12 Production.J Immunol. 2018 Apr 15;200(8):2738-2747. doi: 10.4049/jimmunol.1701614. Epub 2018 Feb 28. J Immunol. 2018. PMID: 29491009 Free PMC article.
-
Enhancement of cytokine-driven NK cell IFN-γ production after vaccination of HCMV infected Africans.Eur J Immunol. 2017 Jun;47(6):1040-1050. doi: 10.1002/eji.201746974. Epub 2017 Apr 24. Eur J Immunol. 2017. PMID: 28383105 Free PMC article.
-
Human Cytokine-Induced Memory-Like Natural Killer Cells.J Innate Immun. 2015;7(6):563-71. doi: 10.1159/000382019. Epub 2015 Apr 30. J Innate Immun. 2015. PMID: 25924651 Free PMC article. Review.
-
Cytokine-Induced Memory-Like NK Cells: From the Basics to Clinical Applications.Front Immunol. 2022 May 4;13:884648. doi: 10.3389/fimmu.2022.884648. eCollection 2022. Front Immunol. 2022. PMID: 35603208 Free PMC article. Review.
Cited by
-
AI-guided discovery of the invariant host response to viral pandemics.EBioMedicine. 2021 Jun;68:103390. doi: 10.1016/j.ebiom.2021.103390. Epub 2021 Jun 11. EBioMedicine. 2021. PMID: 34127431 Free PMC article.
-
Regulation of the human NK cell compartment by pathogens and vaccines.Clin Transl Immunology. 2021 Jan 18;10(1):e1244. doi: 10.1002/cti2.1244. eCollection 2021. Clin Transl Immunology. 2021. PMID: 33505682 Free PMC article. Review.
-
NK Cell Memory to Cytomegalovirus: Implications for Vaccine Development.Vaccines (Basel). 2020 Jul 20;8(3):394. doi: 10.3390/vaccines8030394. Vaccines (Basel). 2020. PMID: 32698362 Free PMC article. Review.
-
Relationship between Humoral Response in COVID-19 and Seasonal Influenza Vaccination.Vaccines (Basel). 2022 Sep 27;10(10):1621. doi: 10.3390/vaccines10101621. Vaccines (Basel). 2022. PMID: 36298486 Free PMC article.
-
Characterization of long non-coding RNA and messenger RNA profiles in laryngeal cancer by weighted gene co-expression network analysis.Aging (Albany NY). 2019 Nov 18;11(22):10074-10099. doi: 10.18632/aging.102419. Epub 2019 Nov 18. Aging (Albany NY). 2019. PMID: 31739287 Free PMC article.
References
-
- Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. - PubMed
-
- Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nature reviews Immunology. 2016;16:112–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

