Extracellular Vesicles in the Blood of Dogs with Cancer-A Preliminary Study
- PMID: 31430895
- PMCID: PMC6720862
- DOI: 10.3390/ani9080575
Extracellular Vesicles in the Blood of Dogs with Cancer-A Preliminary Study
Abstract
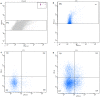
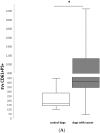
Extracellular vesicles (EVs) are a heterogeneous population of submicron-sized structures released during the activation, proliferation, or apoptosis of various types of cells. Due to their size, their role in cell-to-cell communication in cancer is currently being discussed. In blood, the most abundant population of EVs is platelet-derived EVs (PEVs). The aim of this study was to estimate the absolute number and the origin of EVs in the blood of healthy dogs and of dogs with various types of cancer. The EV absolute number and cellular origin were examined by flow cytometry technique. EVs were classified on the basis of surface annexin V expression (phosphatidylserine PS+) and co-expression of specific cellular markers (CD61, CD45, CD3, CD21). The number of PEVs was significantly higher in dogs with cancer (median: 409/µL, range: 42-2748/µL vs. median: 170/µL, range: 101-449/µL in controls). The numbers of EVs derived from leukocytes (control median: 86/µL, range: 40-240/µL; cancer median: 443/µL, range: 44-3 352/µL) and T cells (control median: 5/µL, range: 2-66/µL; cancer median: 108/µL, range: 3-1735/µL) were higher in dogs with neoplasia compared to healthy controls. The estimation of PEV and leukocyte-derived EV counts may provide a useful biological marker in dogs with cancer.
Keywords: cancer; canine; flow cytometer; platelet microparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Platelet-Released Extracellular Vesicle Characteristics Differ in Chronic and in Acute Heart Disease.Thromb Haemost. 2023 Sep;123(9):892-903. doi: 10.1055/s-0043-57017. Epub 2023 Apr 19. Thromb Haemost. 2023. PMID: 37075787
-
Processing methods and storage duration impact extracellular vesicle counts in red blood cell units.Blood Adv. 2020 Nov 10;4(21):5527-5539. doi: 10.1182/bloodadvances.2020001658. Blood Adv. 2020. PMID: 33166402 Free PMC article.
-
Expression of Tissue Factor and Platelet/Leukocyte Markers on Extracellular Vesicles Reflect Platelet-Leukocyte Interaction in Severe COVID-19.Int J Mol Sci. 2023 Nov 28;24(23):16886. doi: 10.3390/ijms242316886. Int J Mol Sci. 2023. PMID: 38069209 Free PMC article.
-
The biology of extracellular vesicles with focus on platelet microparticles and their role in cancer development and progression.Tumour Biol. 2016 Nov;37(11):14391-14401. doi: 10.1007/s13277-016-5358-6. Epub 2016 Sep 15. Tumour Biol. 2016. PMID: 27629289 Free PMC article. Review.
-
Extracellular vesicles from activated platelets: a semiquantitative cryo-electron microscopy and immuno-gold labeling study.Platelets. 2017 May;28(3):263-271. doi: 10.1080/09537104.2016.1268255. Epub 2017 Jan 19. Platelets. 2017. PMID: 28102751 Review.
Cited by
-
Canine B Cell Lymphoma- and Leukemia-Derived Extracellular Vesicles Moderate Differentiation and Cytokine Production of T and B Cells In Vitro.Int J Mol Sci. 2022 Aug 29;23(17):9831. doi: 10.3390/ijms23179831. Int J Mol Sci. 2022. PMID: 36077229 Free PMC article.
-
Exosome application in treatment and diagnosis of B-cell disorders: leukemias, multiple sclerosis, and arthritis rheumatoid.Cell Mol Biol Lett. 2022 Sep 5;27(1):74. doi: 10.1186/s11658-022-00377-x. Cell Mol Biol Lett. 2022. PMID: 36064322 Free PMC article. Review.
-
Extracellular Vesicles: Novel Opportunities to Understand and Detect Neoplastic Diseases.Vet Pathol. 2021 May;58(3):453-471. doi: 10.1177/0300985821999328. Epub 2021 Apr 5. Vet Pathol. 2021. PMID: 33813952 Free PMC article. Review.
-
Circulating Melanoma-Derived Extracellular Vesicles: Impact on Melanoma Diagnosis, Progression Monitoring, and Treatment Response.Pharmaceuticals (Basel). 2020 Dec 18;13(12):475. doi: 10.3390/ph13120475. Pharmaceuticals (Basel). 2020. PMID: 33353043 Free PMC article. Review.
-
Serum Extracellular Vesicles Cargo Approach in Bitches with Mammary Tumors.Curr Issues Mol Biol. 2024 Jul 22;46(7):7745-7768. doi: 10.3390/cimb46070459. Curr Issues Mol Biol. 2024. PMID: 39057100 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

