Aging- and obesity-related peri-muscular adipose tissue accelerates muscle atrophy
- PMID: 31442231
- PMCID: PMC6707561
- DOI: 10.1371/journal.pone.0221366
Aging- and obesity-related peri-muscular adipose tissue accelerates muscle atrophy
Abstract
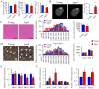
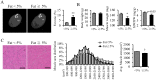
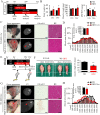
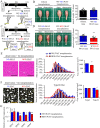
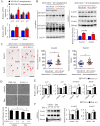
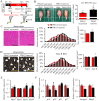
Sarcopenia due to loss of skeletal muscle mass and strength leads to physical inactivity and decreased quality of life. The number of individuals with sarcopenia is rapidly increasing as the number of older people increases worldwide, making this condition a medical and social problem. Some patients with sarcopenia exhibit accumulation of peri-muscular adipose tissue (PMAT) as ectopic fat deposition surrounding atrophied muscle. However, an association of PMAT with muscle atrophy has not been demonstrated. Here, we show that PMAT is associated with muscle atrophy in aged mice and that atrophy severity increases in parallel with cumulative doses of PMAT. We observed severe muscle atrophy in two different obese model mice harboring significant PMAT relative to respective control non-obese mice. We also report that denervation-induced muscle atrophy was accelerated in non-obese young mice transplanted around skeletal muscle with obese adipose tissue relative to controls transplanted with non-obese adipose tissue. Notably, transplantation of obese adipose tissue into peri-muscular regions increased nuclear translocation of FoxO transcription factors and upregulated expression FoxO _targets associated with proteolysis (Atrogin1 and MuRF1) and cellular senescence (p19 and p21) in muscle. Conversely, in obese mice, PMAT removal attenuated denervation-induced muscle atrophy and suppressed upregulation of genes related to proteolysis and cellular senescence in muscle. We conclude that PMAT accumulation accelerates age- and obesity-induced muscle atrophy by increasing proteolysis and cellular senescence in muscle.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Differential expression of perilipin 2 and 5 in human skeletal muscle during aging and their association with atrophy-related genes.Biogerontology. 2015 Jun;16(3):329-40. doi: 10.1007/s10522-014-9549-5. Epub 2015 Jan 6. Biogerontology. 2015. PMID: 25559404
-
Daidzein Inhibits Muscle Atrophy by Suppressing Inflammatory Cytokine- and Muscle Atrophy-Related Gene Expression.Nutrients. 2024 Sep 13;16(18):3084. doi: 10.3390/nu16183084. Nutrients. 2024. PMID: 39339684 Free PMC article.
-
Deletion of atrophy enhancing genes fails to ameliorate the phenotype in a mouse model of spinal muscular atrophy.Neuromuscul Disord. 2014 May;24(5):436-44. doi: 10.1016/j.nmd.2014.02.007. Epub 2014 Feb 25. Neuromuscul Disord. 2014. PMID: 24656734 Free PMC article.
-
Forkhead Box O Signaling Pathway in Skeletal Muscle Atrophy.Am J Pathol. 2022 Dec;192(12):1648-1657. doi: 10.1016/j.ajpath.2022.09.003. Epub 2022 Sep 27. Am J Pathol. 2022. PMID: 36174679 Review.
-
Signalling pathways that mediate skeletal muscle hypertrophy and atrophy.Nat Cell Biol. 2003 Feb;5(2):87-90. doi: 10.1038/ncb0203-87. Nat Cell Biol. 2003. PMID: 12563267 Review.
Cited by
-
The Panax ginseng Berry Extract and Soluble Whey Protein Hydrolysate Mixture Ameliorates Sarcopenia-Related Muscular Deterioration in Aged Mice.Nutrients. 2022 Feb 14;14(4):799. doi: 10.3390/nu14040799. Nutrients. 2022. PMID: 35215448 Free PMC article.
-
Obesity as a premature aging phenotype - implications for sarcopenic obesity.Geroscience. 2022 Jun;44(3):1393-1405. doi: 10.1007/s11357-022-00567-7. Epub 2022 Apr 26. Geroscience. 2022. PMID: 35471692 Free PMC article. Review.
-
Fat Is Consistently Present within the Plantar Muscular Space of the Human Foot-An Anatomical Study.Medicina (Kaunas). 2022 Jan 20;58(2):154. doi: 10.3390/medicina58020154. Medicina (Kaunas). 2022. PMID: 35208480 Free PMC article.
-
Role of Metabolic Stress and Exercise in Regulating Fibro/Adipogenic Progenitors.Front Cell Dev Biol. 2020 Jan 28;8:9. doi: 10.3389/fcell.2020.00009. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32047748 Free PMC article. Review.
-
What's gut got to do with it? The role of the microbiota and inflammation in the development of adiposity and obesity.Immunometabolism (Cobham). 2023 Jul 24;5(3):e00029. doi: 10.1097/IN9.0000000000000029. eCollection 2023 Jul. Immunometabolism (Cobham). 2023. PMID: 37492183 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

