Green Tea Polyphenol (-)-Epigallocatechin Gallate (EGCG) Attenuates Neuroinflammation in Palmitic Acid-Stimulated BV-2 Microglia and High-Fat Diet-Induced Obese Mice
- PMID: 31614951
- PMCID: PMC6834190
- DOI: 10.3390/ijms20205081
Green Tea Polyphenol (-)-Epigallocatechin Gallate (EGCG) Attenuates Neuroinflammation in Palmitic Acid-Stimulated BV-2 Microglia and High-Fat Diet-Induced Obese Mice
Abstract
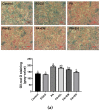
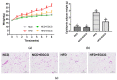
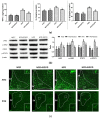
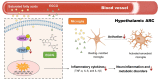
Obesity is closely associated with neuroinflammation in the hypothalamus, which is characterized by over-activated microglia and excessive production of pro-inflammatory cytokines. The present study was aimed at elucidating the effects of (-)-epigallocatechin gallate (EGCG) on palmitic acid-stimulated BV-2 microglia and high-fat-diet-induced obese mice. The results indicated the suppressive effect of EGCG on lipid accumulation, pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) release, and microglial activation in both cellular and high-fat-diet rodent models. These results were associated with lower phosphorylated levels of the janus kinase 2/signal transducers and activators of transcription 3 (JAK2/STAT3) signaling pathway. In conclusion, EGCG can attenuate high-fat-induced hypothalamic inflammation via inhibiting the JAK2/STAT3 signaling pathways in microglia.
Keywords: hypothalamus; inflammation; obesity; saturated fatty acids; tea polyphenols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Effects of (-)-Epigallocatechin Gallate (EGCG) on Energy Expenditure and Microglia-Mediated Hypothalamic Inflammation in Mice Fed a High-Fat Diet.Nutrients. 2018 Nov 5;10(11):1681. doi: 10.3390/nu10111681. Nutrients. 2018. PMID: 30400620 Free PMC article.
-
Epigallocatechin gallate attenuates amyloid β-induced inflammation and neurotoxicity in EOC 13.31 microglia.Eur J Pharmacol. 2016 Jan 5;770:16-24. doi: 10.1016/j.ejphar.2015.11.048. Epub 2015 Nov 28. Eur J Pharmacol. 2016. PMID: 26643169
-
Carbenoxolone ameliorates hepatic lipid metabolism and inflammation in obese mice induced by high fat diet via regulating the JAK2/STAT3 signaling pathway.Int Immunopharmacol. 2019 Sep;74:105498. doi: 10.1016/j.intimp.2019.03.011. Epub 2019 Jun 29. Int Immunopharmacol. 2019. PMID: 31261036
-
Therapeutic Effects of Green Tea Polyphenol (‒)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis.Int J Mol Sci. 2022 Dec 25;24(1):340. doi: 10.3390/ijms24010340. Int J Mol Sci. 2022. PMID: 36613784 Free PMC article. Review.
-
The anti-obesity effects of green tea in human intervention and basic molecular studies.Eur J Clin Nutr. 2014 Oct;68(10):1075-87. doi: 10.1038/ejcn.2014.143. Epub 2014 Jul 30. Eur J Clin Nutr. 2014. PMID: 25074392 Review.
Cited by
-
Phytochemical _targeting of STAT3 Orchestrated Lipid Metabolism in Therapy-Resistant Cancers.Biomolecules. 2020 Jul 28;10(8):1118. doi: 10.3390/biom10081118. Biomolecules. 2020. PMID: 32731620 Free PMC article. Review.
-
Cellular Defensive Mechanisms of Tea Polyphenols: Structure-Activity Relationship.Int J Mol Sci. 2021 Aug 24;22(17):9109. doi: 10.3390/ijms22179109. Int J Mol Sci. 2021. PMID: 34502017 Free PMC article. Review.
-
The Effect of Intragastric Gavage of High Dose Green Tea Extract on Serum Status of Magnesium, Calcium, and Zinc.Asian Pac J Cancer Prev. 2022 Sep 1;23(9):3195-3199. doi: 10.31557/APJCP.2022.23.9.3195. Asian Pac J Cancer Prev. 2022. PMID: 36172684 Free PMC article.
-
Epigallocatechin Gallate Protects against Hypoxia-Induced Inflammation in Microglia via NF-κB Suppression and Nrf-2/HO-1 Activation.Int J Mol Sci. 2022 Apr 4;23(7):4004. doi: 10.3390/ijms23074004. Int J Mol Sci. 2022. PMID: 35409364 Free PMC article.
-
Epigallocatechin Gallate (EGCG) Promotes the Immune Function of Ileum in High Fat Diet Fed Mice by Regulating Gut Microbiome Profiling and Immunoglobulin Production.Front Nutr. 2021 Sep 20;8:720439. doi: 10.3389/fnut.2021.720439. eCollection 2021. Front Nutr. 2021. PMID: 34616764 Free PMC article.
References
-
- De Souza C.T., Araujo E.P., Bordin S., Ashimine R., Zollner R.L., Boschero A.C., Saad M.J.A., Velloso L.A. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

