Make the right measurement: Discovery of an allosteric inhibition site for p300-HAT
- PMID: 31649965
- PMCID: PMC6800282
- DOI: 10.1063/1.5119336
Make the right measurement: Discovery of an allosteric inhibition site for p300-HAT
Abstract
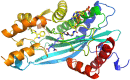
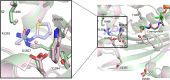
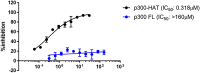
Histone acetyltransferases (HATs) and histone deacetylases (HDACs) catalyze the dynamic and reversible acetylation of proteins, an epigenetic regulatory mechanism associated with multiple cancers. Indeed, HDAC inhibitors are already approved in the clinic. The HAT paralogs p300 and CREB-binding protein (CBP) have been implicated in human pathological conditions including several hematological malignancies and androgen receptor-positive prostate cancer. Others have reported CoA-competitive inhibitors of p300 and CBP with cell-based activity. Here, we describe 2 compounds, CPI-076 and CPI-090, discovered through p300-HAT high throughput screening screening, which inhibit p300-HAT via binding at an allosteric site. We present the high resolution (1.7 and 2.3 Å) co-crystal structures of these molecules bound to a previously undescribed allosteric site of p300-HAT. Derivatization yielded actionable structure-activity relationships, but the full-length enzymatic assay demonstrated that this allosteric HAT inhibitor series was artifactual, inhibiting only the HAT domain of p300 with no effect on the full-length enzyme.
© 2019 Author(s).
Figures

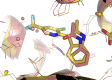

Similar articles
-
Discovery of a selective catalytic p300/CBP inhibitor that _targets lineage-specific tumours.Nature. 2017 Oct 5;550(7674):128-132. doi: 10.1038/nature24028. Epub 2017 Sep 27. Nature. 2017. PMID: 28953875 Free PMC article.
-
Epigenetic Modulation Using Small Molecules - _targeting Histone Acetyltransferases in Disease.Curr Med Chem. 2017;24(37):4121-4150. doi: 10.2174/0929867324666170223153115. Curr Med Chem. 2017. PMID: 28240169
-
Activation of p300 histone acetyltransferase activity and acetylation of the androgen receptor by bombesin in prostate cancer cells.Oncogene. 2006 Mar 30;25(14):2011-21. doi: 10.1038/sj.onc.1209231. Oncogene. 2006. PMID: 16434977
-
Modulating epigenetic HAT activity for reinstating acetylation homeostasis: A promising therapeutic strategy for neurological disorders.Pharmacol Ther. 2016 Oct;166:106-22. doi: 10.1016/j.pharmthera.2016.07.001. Epub 2016 Jul 10. Pharmacol Ther. 2016. PMID: 27411674 Review.
-
Regulation of protein turnover by acetyltransferases and deacetylases.Biochimie. 2008 Feb;90(2):306-12. doi: 10.1016/j.biochi.2007.06.009. Epub 2007 Jul 1. Biochimie. 2008. PMID: 17681659 Review.
Cited by
-
Active components and molecular mechanisms of Sagacious Confucius' Pillow Elixir to treat cognitive impairment based on systems pharmacology.Aging (Albany NY). 2023 Jul 30;15(14):7278-7307. doi: 10.18632/aging.204912. Epub 2023 Jul 30. Aging (Albany NY). 2023. PMID: 37517091 Free PMC article.
-
MOF upregulates the estrogen receptor α signaling pathway by its acetylase activity in hepatocellular carcinoma.Cancer Sci. 2021 May;112(5):1865-1877. doi: 10.1111/cas.14836. Epub 2021 Mar 30. Cancer Sci. 2021. PMID: 33544437 Free PMC article.
-
N-1,2,3-Triazole-isatin derivatives: anti-proliferation effects and _target identification in solid tumour cell lines.RSC Med Chem. 2022 Jun 10;13(8):970-977. doi: 10.1039/d2md00044j. eCollection 2022 Aug 17. RSC Med Chem. 2022. PMID: 36092141 Free PMC article.
-
The Biological Significance of _targeting Acetylation-Mediated Gene Regulation for Designing New Mechanistic Tools and Potential Therapeutics.Biomolecules. 2021 Mar 18;11(3):455. doi: 10.3390/biom11030455. Biomolecules. 2021. PMID: 33803759 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

