Stretch-activated current in human atrial myocytes and Na+ current and mechano-gated channels' current in myofibroblasts alter myocyte mechanical behavior: a computational study
- PMID: 31653259
- PMCID: PMC6814973
- DOI: 10.1186/s12938-019-0723-5
Stretch-activated current in human atrial myocytes and Na+ current and mechano-gated channels' current in myofibroblasts alter myocyte mechanical behavior: a computational study
Abstract
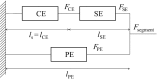
Background: The activation of stretch-activated channels (SACs) in cardiac myocytes, which changes the phases of action potential repolarization, is proven to be highly efficient for the conversion of atrial fibrillation. The expression of Na+ current in myofibroblasts (Mfbs) regenerates myocytes' action potentials, suggesting that Mfbs play an active role in triggering cardiac rhythm disturbances. Moreover, the excitation of mechano-gated channels (MGCs) in Mfbs depolarizes their membrane potential and contributes to the increased risk of post-infarct arrhythmia. Although these electrophysiological mechanisms have been largely known, the roles of these currents in cardiac mechanics are still debated. In this study, we aimed to investigate the mechanical influence of these currents via mathematical modeling. A novel mathematical model was developed by integrating models of human atrial myocyte (including the stretch-activated current, Ca2+-force relation, and mechanical behavior of a single segment) and Mfb (including our formulation of Na+ current and mechano-gated channels' current). The effects of the changes in basic cycle length, number of coupled Mfbs and intercellular coupling conductance on myocyte mechanical properties were compared.
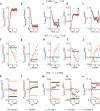
Results: Our results indicated that these three currents significantly regulated myocyte mechanical parameters. In isosarcometric contraction, these currents increased segment force by 13.8-36.6% and dropped element length by 12.1-31.5%. In isotonic contraction, there are 2.7-5.9% growth and 0.9-24% reduction. Effects of these currents on the extremum of myocyte mechanical parameters become more significant with the increase of basic cycle length, number of coupled Mfbs and intercellular coupling conductance.
Conclusions: The results demonstrated that stretch-activated current in myocytes and Na+ current and mechano-gated channels' current in Mfbs significantly influenced myocyte mechanical behavior and should be considered in future cardiac mechanical mathematical modeling.
Keywords: Mathematical modeling; Mechano-gated channels (MGCs); Myocyte mechanics; Myofibroblast–myocyte (Mfb–M) coupling; Stretch-activated channels (SACs); Voltage-gated sodium channels (VGSCs).
Conflict of interest statement
The authors declare that they have no competing of interests.
Figures
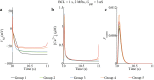
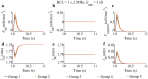
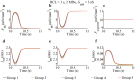
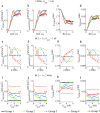
Similar articles
-
Effects of Na+ Current and Mechanogated Channels in Myofibroblasts on Myocyte Excitability and Repolarization.Comput Math Methods Med. 2016;2016:6189374. doi: 10.1155/2016/6189374. Epub 2016 Nov 17. Comput Math Methods Med. 2016. PMID: 27980607 Free PMC article.
-
An integrative appraisal of mechano-electric feedback mechanisms in the heart.Prog Biophys Mol Biol. 2017 Nov;130(Pt B):404-417. doi: 10.1016/j.pbiomolbio.2017.08.008. Epub 2017 Aug 26. Prog Biophys Mol Biol. 2017. PMID: 28851517 Free PMC article.
-
Single mechano-gated channels activated by mechanical deformation of acutely isolated cardiac fibroblasts from rats.Acta Physiol (Oxf). 2010 Jul 1;199(3):277-92. doi: 10.1111/j.1748-1716.2010.02086.x. Epub 2010 Jan 25. Acta Physiol (Oxf). 2010. PMID: 20102342
-
Role of stretch-activated channels on the stretch-induced changes of rat atrial myocytes.Prog Biophys Mol Biol. 2006 Jan-Apr;90(1-3):186-206. doi: 10.1016/j.pbiomolbio.2005.06.003. Epub 2005 Jul 7. Prog Biophys Mol Biol. 2006. PMID: 16043213 Review.
-
Are Interactions between Epicardial Adipose Tissue, Cardiac Fibroblasts and Cardiac Myocytes Instrumental in Atrial Fibrosis and Atrial Fibrillation?Cells. 2021 Sep 21;10(9):2501. doi: 10.3390/cells10092501. Cells. 2021. PMID: 34572150 Free PMC article. Review.
Cited by
-
Platelet-Rich Plasma Modulates Gap Junction Functionality and Connexin 43 and 26 Expression During TGF-β1-Induced Fibroblast to Myofibroblast Transition: Clues for Counteracting Fibrosis.Cells. 2020 May 12;9(5):1199. doi: 10.3390/cells9051199. Cells. 2020. PMID: 32408529 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

