Peripheral nerve injury-induced alterations in VTA neuron firing properties
- PMID: 31685030
- PMCID: PMC6827252
- DOI: 10.1186/s13041-019-0511-y
Peripheral nerve injury-induced alterations in VTA neuron firing properties
Abstract
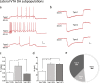
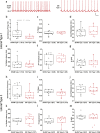
The ventral tegmental area (VTA) is one of the main brain regions harboring dopaminergic (DA) neurons, and plays important roles in reinforcement and motivation. Recent studies have indicated that DA neurons not only respond to rewarding stimuli, but also to noxious stimuli. Furthermore, VTA DA neurons undergo plasticity during chronic pain. Lateral and medial VTA neurons project to different brain areas, and have been characterized via their distinct electrophysiological properties. In this study, we characterized electrophysiological properties of lateral and medial VTA DA neurons using DAT-cre reporter mice, and examined their plasticity during neuropathic pain states. We observed various DA subpopulations in both the lateral and medial VTA, as defined by action potential firing patterns, independently of synaptic inputs. Our results demonstrated that lateral and medial VTA DA neurons undergo differential plasticity after peripheral nerve injury that leads to neuropathic pain. However, these changes only reside in specific DA subpopulations. This study suggests that lateral and medial VTA DA neurons are differentially affected during neuropathic pain conditions, and emphasizes the importance of subpopulation specificity when _targeting VTA DA neurons for treatment of neuropathic pain.
Keywords: Brain circuits; Dopamine; Pain; Prefrontal cortex; Ventral tegmental area.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Determination of circuit-specific morphological adaptations in ventral tegmental area dopamine neurons by chronic morphine.Mol Brain. 2019 Feb 8;12(1):10. doi: 10.1186/s13041-019-0435-6. Mol Brain. 2019. PMID: 30736837 Free PMC article.
-
A subset of ventral tegmental area dopamine neurons responds to acute ethanol.Neuroscience. 2015 Apr 2;290:649-58. doi: 10.1016/j.neuroscience.2014.12.081. Epub 2015 Feb 7. Neuroscience. 2015. PMID: 25660505 Free PMC article.
-
Prenatal Ethanol Exposure Persistently Alters Endocannabinoid Signaling and Endocannabinoid-Mediated Excitatory Synaptic Plasticity in Ventral Tegmental Area Dopamine Neurons.J Neurosci. 2017 Jun 14;37(24):5798-5808. doi: 10.1523/JNEUROSCI.3894-16.2017. Epub 2017 May 5. J Neurosci. 2017. PMID: 28476947 Free PMC article.
-
Diversity of Dopaminergic Neural Circuits in Response to Drug Exposure.Neuropsychopharmacology. 2016 Sep;41(10):2424-46. doi: 10.1038/npp.2016.32. Epub 2016 Mar 3. Neuropsychopharmacology. 2016. PMID: 26934955 Free PMC article. Review.
-
Stress-induced plasticity and functioning of ventral tegmental dopamine neurons.Neurosci Biobehav Rev. 2020 Jan;108:48-77. doi: 10.1016/j.neubiorev.2019.10.015. Epub 2019 Oct 27. Neurosci Biobehav Rev. 2020. PMID: 31666179 Review.
Cited by
-
Role of Glutamatergic Projections from Lateral Habenula to Ventral Tegmental Area in Inflammatory Pain-Related Spatial Working Memory Deficits.Biomedicines. 2023 Mar 8;11(3):820. doi: 10.3390/biomedicines11030820. Biomedicines. 2023. PMID: 36979799 Free PMC article.
-
Spatial Distribution of Neurons Expressing Single, Double, and Triple Molecular Characteristics of Glutamatergic, Dopaminergic, or GABAergic Neurons in the Mouse Ventral Tegmental Area.J Mol Neurosci. 2023 Jun;73(6):345-362. doi: 10.1007/s12031-023-02121-2. Epub 2023 May 27. J Mol Neurosci. 2023. PMID: 37243808
-
Association between Chronic Pain and Alterations in the Mesolimbic Dopaminergic System.Brain Sci. 2020 Oct 2;10(10):701. doi: 10.3390/brainsci10100701. Brain Sci. 2020. PMID: 33023226 Free PMC article. Review.
-
An Ascending Excitatory Circuit from the Dorsal Raphe for Sensory Modulation of Pain.J Neurosci. 2024 Jan 24;44(4):e0869232023. doi: 10.1523/JNEUROSCI.0869-23.2023. J Neurosci. 2024. PMID: 38124016 Free PMC article.
-
Dysregulated neuromodulation in the anterior cingulate cortex in chronic pain.Front Pharmacol. 2023 Oct 25;14:1289218. doi: 10.3389/fphar.2023.1289218. eCollection 2023. Front Pharmacol. 2023. PMID: 37954846 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

