Antibodies from the Sera of Multiple Sclerosis Patients Efficiently Hydrolyze Five Histones
- PMID: 31731780
- PMCID: PMC6920934
- DOI: 10.3390/biom9110741
Antibodies from the Sera of Multiple Sclerosis Patients Efficiently Hydrolyze Five Histones
Abstract
It is known that intranuclear histones can be pernicious after entering to the extracellular space. In addition, the immunization of animals with exogenous histones leads to systemic inflammatory and toxic reactions. Abzymes-autoantibodies with enzymatic activities-are the distinctive feature of autoimmune diseases and they can be especially dangerous to humans. Here, electrophoretically homogeneous IgGs were isolated from sera of patients with multiple sclerosis (MS) by chromatography on several affinity sorbents. We present evidence that sera of all MS patients contain autoantibodies against histones and 73% of IgGs purified from the sera of 59 MS patients efficiently hydrolyze from one to five histones: H1, H2a, H2b, H3, and H4. The relative average efficiency of the histones hydrolysis was ~3.9-fold higher than that for healthy donors. The relative average activity of IgGs depends on the type of MS and decreased approximately in the following order: debut of MS, secondary progressive multiple sclerosis, remitting multiple sclerosis, remittent progressive multiple sclerosis. Similar to proteolytic abzymes of patients with several autoimmune diseases, histone-hydrolyzing IgGs from MS patients were inhibited in the presence of specific inhibitors of serine and of metal-dependent proteases, but an unexpected significant inhibition of the activity by inhibitors of thiol-like and especially acidic proteases was observed. Since IgGs can efficiently hydrolyze histones, a negative role of abzymes in the development of MS cannot be excluded.
Keywords: catalytic IgGs; human blood antibodies; hydrolysis of human histones; multiple sclerosis patients.
Conflict of interest statement
the authors declare the absence of competing financial interests.
Figures
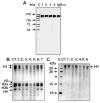
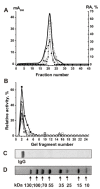
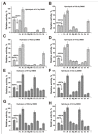
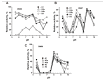
Similar articles
-
Multiple Sclerosis: Enzymatic Cross Site-Specific Hydrolysis of H1 Histone by IgGs against H1, H2A, H2B, H3, H4 Histones, and Myelin Basic Protein.Biomolecules. 2021 Aug 2;11(8):1140. doi: 10.3390/biom11081140. Biomolecules. 2021. PMID: 34439806 Free PMC article.
-
Antibodies from the sera of HIV-infected patients efficiently hydrolyze all human histones.J Mol Recognit. 2016 Aug;29(8):346-62. doi: 10.1002/jmr.2534. Epub 2016 Jan 22. J Mol Recognit. 2016. PMID: 26799177
-
Multiple Sclerosis: Enzymatic Cross Site-Specific Recognition and Hydrolysis of H2A Histone by IgGs against H2A, H1, H2B, H3 Histones, Myelin Basic Protein, and DNA.Biomedicines. 2022 Aug 3;10(8):1876. doi: 10.3390/biomedicines10081876. Biomedicines. 2022. PMID: 36009424 Free PMC article.
-
Autoantibodies to factor VIII with catalytic activity.Autoimmun Rev. 2003 Jan;2(1):30-5. doi: 10.1016/s1568-9972(02)00126-x. Autoimmun Rev. 2003. PMID: 12848973 Review.
-
[Achievements and prospects of clinical abzymology].Vestn Ross Akad Med Nauk. 2005;(9):38-43. Vestn Ross Akad Med Nauk. 2005. PMID: 16250330 Review. Russian.
Cited by
-
Cell Differentiation and Proliferation in the Bone Marrow and Other Organs of 2D2 Mice during Spontaneous Development of EAE Leading to the Production of Abzymes.Molecules. 2022 Mar 28;27(7):2195. doi: 10.3390/molecules27072195. Molecules. 2022. PMID: 35408594 Free PMC article.
-
IgGs-Abzymes from the Sera of Patients with Multiple Sclerosis Recognize and Hydrolyze miRNAs.Int J Mol Sci. 2021 Mar 10;22(6):2812. doi: 10.3390/ijms22062812. Int J Mol Sci. 2021. PMID: 33802122 Free PMC article.
-
Some structural features of the peptide profile of myelin basic protein-hydrolyzing antibodies in schizophrenic patients.PeerJ. 2023 Jul 6;11:e15584. doi: 10.7717/peerj.15584. eCollection 2023. PeerJ. 2023. PMID: 37431466 Free PMC article.
-
Multiple Sclerosis: Enzymatic Cross Site-Specific Recognition and Hydrolysis of H3 Histone by IgGs against H3, H1, H2A, H2B, H4 Histones, Myelin Basic Protein, and DNA.Biomedicines. 2022 Oct 21;10(10):2663. doi: 10.3390/biomedicines10102663. Biomedicines. 2022. PMID: 36289924 Free PMC article.
-
Extreme Diversity of IgGs Against Histones, DNA, and Myelin Basic Protein in the Cerebrospinal Fluid and Blood of Patients with Multiple Sclerosis.Biomolecules. 2020 Apr 18;10(4):630. doi: 10.3390/biom10040630. Biomolecules. 2020. PMID: 32325782 Free PMC article.
References
-
- Lerner R.A., Tramontano A. Antibodies as enzymes. Trends Bioch. Sci. 1987;12:427–438. doi: 10.1016/0968-0004(87)90208-8. - DOI
-
- David B.S. In: Catalytic Antibodies. Keinan E., editor. Wiley-VCH Verlag GmbH and Co. KgaA; Weinheim, Germany: 2005. pp. 1–586.
-
- Nevinsky G.A., Buneva V.N. Natural catalytic antibodies–abzymes. In: Keinan E., editor. Catalytic Antibodies. VCH-Wiley Press; Weinheim, Germany: 2005. pp. 505–569.
-
- Nevinsky G.A. Natural catalytic antibodies in norm and in autoimmune diseases. In: Brenner K.J., editor. Autoimmune Diseases: Symptoms, Diagnosis and Treatment. Nova Science Publishers Inc.; New York, NY, USA: 2010. pp. 1–107.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

